Table 1.
Study designs
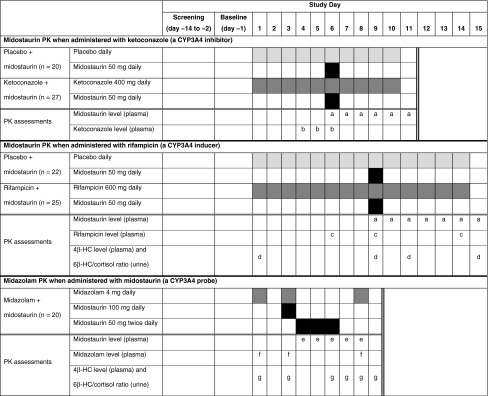
Black highlight indicates midostaurin. Medium gray highlight indicates CYP3A4 inhibitor, inducer, or probe. Light gray highlight indicates placebo. Double vertical lines indicate the end of a given study
4β-HC 4β-hydroxycholesterol, 6β-HC 6β-hydroxycortisol, CYP3A4 cytochrome P450 3A4, PK pharmacokinetics
aBlood samples for the determination of midostaurin PK were collected predose and at 0.5, 1, 1.5, 2, 3, 4, 6, 8, 12, 24, 36, 48, 72, 96, and 120 h after midostaurin dosing on day 6 (ketoconazole study) and day 9 (rifampicin study). In the rifampicin study, an additional sample was taken at 144 h after midostaurin dosing on day 9
bOn days 4–6, blood samples were collected before the morning dose to measure ketoconazole levels
cOn days 6, 9, and 14, blood samples were collected for rifampicin assessment before the evening rifampicin dose
dOn days 1 (baseline), 9 (before midostaurin treatment), 11, and 15, levels of 4β-HC in plasma and the ratio of 6β-HC/cortisol in urine were assessed
eBlood samples for PK determination of midostaurin and its metabolites (CGP62221 and CGP52421) were taken before the morning dose of midostaurin on days 4–6 and before midazolam administration on days 7 and 8
fBlood samples for the determination of midazolam PK were collected before dose and at 0.25, 0.5, 1, 1.5, 1.75, 2, 3, 4, 6, 8, and 10 h after midazolam dosing on days 1, 3, and 8
gBlood samples and urine samples were taken on days 1, 3, 6, 7, 8, and 9 to measure 4β-HC in plasma and the ratio of 6β-HC/cortisol in urine, respectively
