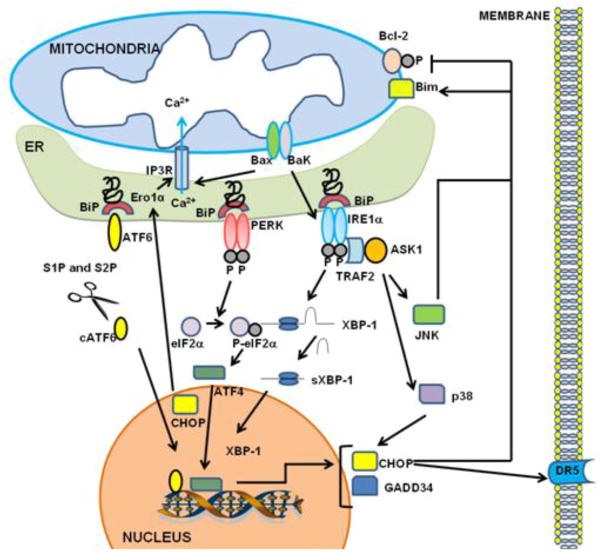
Figure 1. Endoplasmic reticulum (ER) stress signaling.
The ER stress response is mediated by three sensors located at the ER membrane: IRE1α, ATF6 and PERK. Accumulation of unfolded protein recruits BiP to the ER lumen and its dissociation from IRE1α, ATF6 and PERK leads to their activation. Upon dimerization and autophosphorylation, IRE1α splices XBP-1 mRNA, which adjusts the reading frame to allow translation of an active transcriptional factor XBP-1. XBP-1 up-regulates UPR genes encoding ER chaperones and components of the ERAD machinery. IRE1α can also recruit TRAF2 and ASK1, leading to downstream activation of JNK and p38 MAPK. Activated JNK translocates to the mitochondrial membrane and promotes (a) activation of Bim and (b) inhibition of Bcl-2, whereas p38 MAPK phosphorylates and activates CHOP. CHOP can induce transcriptional activation of genes that contribute to cell death, including Ero1α (hyperoxidizes the ER and activates IP3R) and DR5 (promotes caspase 8-dependent cell death). Bax and Bak can also (a) bind to and activate IRE1α and (b) induce release of Ca2+ from the ER. PERK phosphorylates elF2α and attenuates protein translation. However, translation of selected mRNAs is favored under these conditions, including ATF4, which then induces expression of CHOP and GADD34. Activated ATF6 translocates to the Golgi where its cytosolic domain is cleaved by the proteases, S1P and S2P. The cleaved ATF6 fragment forms an active transcriptional factor that mediates expression of several components important for protein folding, degradation, and ER expansion.