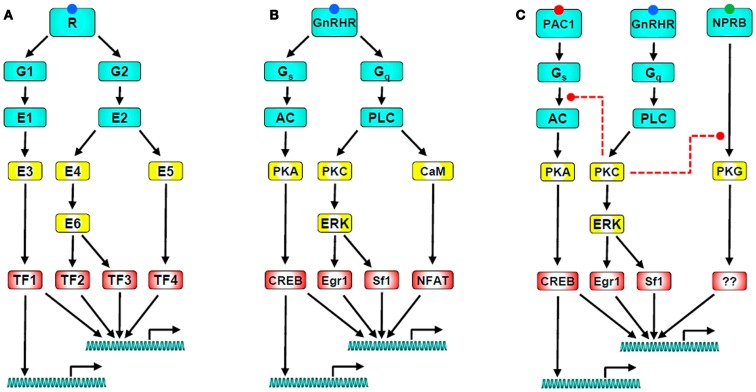
Figure 1.
GnRH receptor signaling networks. (A) Illustrates a generic signaling network in which a GPCR activates two heterotrimeric G-proteins (G1 and G2) which activate their cognate effectors (E1 and E2). These directly or indirectly activate down-stream effectors that influence a range of target proteins including transcription factors (TF1-4). The transcription factors then act (typically in combination) to influence expression of numerous target genes. Note that the network has multiple sites for divergence and convergence. (B) Shows a GnRH signaling network with the same architecture; The GnRHR activates Gs and Gq leading to activation of adenylyl cyclase (AC) and phospholipase C (PLC). AC generates cAMP, stimulating PKA which activates the transcription factor CREB. PLC leads to activation of PKC, driving activation of ERK and of ERK-dependent transcription via Sf-1 and Egr-1. It also elevates the cytoplasmic Ca2+ concentration, driving activation of calmodulin and its targets, including calcineurin which leads to activation of the Ca2+-dependent transcription factor NFAT. This cartoon is clearly a vast oversimplification as important effectors (including calmodulin-dependent kinases, JNK, p38, and nitric oxide synthase) are not included. Perhaps more importantly, it also excludes signal dynamics and heterologous regulation, both of which are important for control of gonadotropes. A simple example of the latter is given in (C) which includes the PAC1 receptor as a mediator of PACAP-stimulated AC activation, and the NPRB receptor as a mediator of CNP-stimulated cGMP accumulation and consequent protein kinase G (PKG) activation. GnRH can cause PKC-mediated inhibition of PACAP-stimulated cAMP accumulation and of CNP-stimulated cGMP accumulation (as indicated by the dashed red lines), raising the possibility that its predominant effect is actually inhibition of these pathways in gonadotropes exposed to autocrine or paracrine stimulation of PAC1 and NPRB. Finally, when considering signal dynamics, it is important to recognize: (a) that GnRH is secreted in pulses, (b) that the responses illustrated have distinct kinetics, (c) that the kinetics of convergent pathways are important determinants of GnRH pulse frequency-response relationships, and (d) that GnRH influences the expression of many genes encoding components of the GnRHR signaling pathways, with transcription-dependent feedback loops supporting an adaptive signaling network.