Abstract
Background
Atrial premature complexes (APC) are among the most frequently encountered electrocardiographic abnormalities. However, their prognostic value among healthy individuals is unclear. This study aimed to clarify the role of APC in predicting cardiovascular events in a large Japanese community cohort using long-term follow-up data.
Methods
National Integrated Project for Prospective Observation of Non-communicable Disease And its Trends in the Aged, 1990-2005, (NIPPON DATA 90) was a large cohort study of cardiovascular disease (CVD) in Japan. A total of 7692 otherwise healthy participants with no history of myocardial infarction, stroke, atrial fibrillation, or atrial flutter were enrolled (men, 41.5%; mean age, 52.5 ± 13.7 years).
Results
A total of 64 (0.8%) participants had at least one beat of APC on screening 12-lead electrocardiogram. During the follow-up of 14.0 ± 2.9 years (total, 107,474 patient-years), 338 deaths occurred due to CVD. The association between APC and CVD outcome was assessed using Cox proportional hazard models. Cox regression analysis revealed that the presence of APC was an independent predictor for CVD deaths (HR: 2.03, 95% CI: 1.12–3.66, P = 0.019). The association of APC on CVD death was more evident in participants with hypertension (P-value for interaction, 0.03).
Conclusions
APC recorded during the screening electrocardiogram are significantly associated with an increased risk of CVD deaths in a Japanese community-dwelling population and are a strong prognostic factor for hypertensive participants.
Introduction
Cardiovascular disease (CVD) is the largest single cause of death, accounting for approximately 30% of all deaths worldwide[1]. Electrocardiographic (ECG) screening has been performed in various situations to identify the early signs of CVD, and atrial premature complexes (APC) are among the most frequently recorded abnormal findings[2,3]. Nevertheless, APC detected in participants with apparently normal hearts have been regarded as a relatively benign phenomenon and do not contribute to prognostic risk stratification.
However, with the elaboration of cohort studies and large-scale registries in recent years, the potential hazards of premature complexes associated with various cardiac conditions have gradually been recognized. Frequent APC have been shown to be associated with a risk of atrial fibrillation, which subsequently leads to an increased risk of cerebral infarction[4-6]. Previous studies were limited in terms of being mostly derived from studies that used a 24-hour ambulatory ECG to evaluate relatively high-risk populations such as the elderly or those with certain risk factors, including hypertension. Furthermore, although atrial fibrillation leading to cerebral infarction is a major clinical event, the association of APC and actual mortality remains unclear.
In this study, we sought to investigate the prognostic value of APC recorded during the screening ECG in a large community cohort study that enabled analyses of long-term CVD outcomes in Japan.
Methods
Participants
The cohort studies of the National Survey on Circulatory Disorders, Japan, are referred to as NIPPON DATA (National Integrated Project for Prospective Observation of Non-communicable Disease And its Trends in the Aged), including a total of 2 cohort studies. The details of this cohort have been reported previously[7-19]. The baseline surveys were performed in 1980 and 1990 (NIPPON DATA 80 and NIPPON DATA 90). In the present study, we analyzed the data from NIPPON DATA 90, because the baseline survey of NIPPON DATA 80 recorded VPC and APC as a single category that was not further discriminated.
A total of 8383 healthy participants (3504 men and 4879 women, ≥30 years of age) from 300 randomly selected districts throughout Japan were followed from 1990 until 2005. The total population aged ≥30 years in these selected districts was 10,956; therefore, the participation rate was 76.5% before exclusion for the reasons given below. The survey consisted of history taking, physical examinations, blood tests, standard 12-lead ECG recordings, and self-administered questionnaires on lifestyle.
A total of 691 participants were excluded for the following reasons: history of a known vascular condition, such as myocardial infarction or stroke (n = 248); presence of atrial fibrillation or flutter (n = 54); some missing information at the baseline survey (n = 120); and absence of a permanent address that was needed to link to these vital statistical records (n = 269). The remaining 7692 otherwise healthy participants were included in our study (Figure 1).
Figure 1. Study cohort creation.
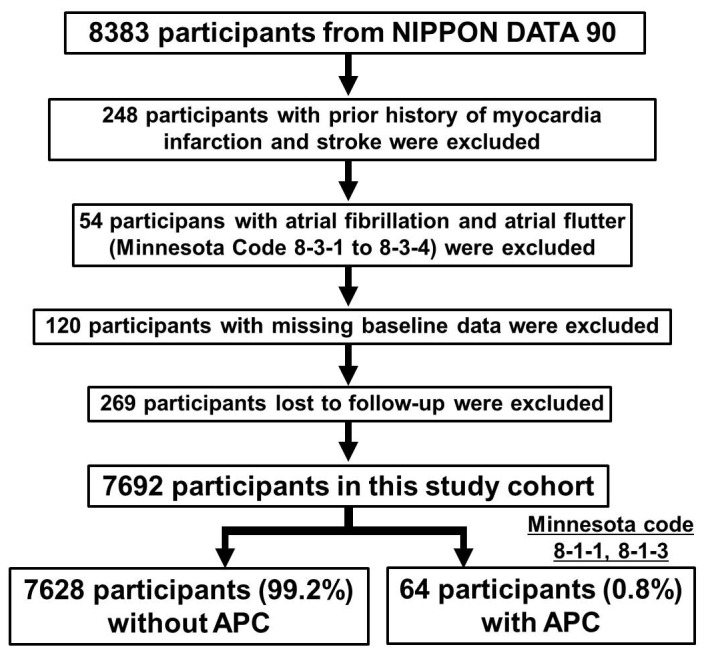
APC, atrial premature complexes.
Baseline Examination
A standard 12-lead ECG was recorded in the resting supine position. Each ECG was reviewed independently by 2 trained coders according to the Minnesota Code (MC), as described previously[7-9,12,17]. Codes in agreement were accepted, whereas discordant results were adjudicated by a panel of study epidemiologists and cardiologists. Participants with at least one beat of APC (more than 10% of recorded QRS complexes) were determined as having APC; they were further classified according to the presence of APC (MC 8-1-1 or MC 8-1-3). Additional ECG findings examined included Q-wave abnormality (MC 1-1, 1-2, 1-3), QRS axis deviation (MC 2-1, 2-2, 2-3), high R wave (MC 3-1 to 3-4), ST depression (MC 4-1 to 4-4), T-wave abnormality (MC 5-1 to 5-5), intraventricular conduction block (MC 7-1-1 to 7-8), P-wave abnormality (MC 9-3-1 or 9-3-2), clockwise or counterclockwise rotation (MC 9-4-1 or 9-4-2), and ventricular premature complexes (MC 8-1-2 or MC 8-1-3).
Baseline blood pressures were measured by trained observers using a standard mercury sphygmomanometer on the right arm with the subject in a seated position. Hypertension was defined as systolic blood pressure of 140 mmHg or higher, diastolic blood pressure of 90 mmHg or higher, use of antihypertensive agents, or any combination of these. Non-fasting blood samples were centrifuged within 60 minutes of collection and were stored at -70°C until analysis. The blood samples were analyzed at the central laboratory (SRL, Tokyo, Japan) using established methods, as described previously[10,14-16]. Hypercholesterolemia was defined as serum total cholesterol ≥240 mg/dL, the use of medications for hypercholesterolemia, or both. Plasma glucose was also measured enzymatically. Diabetes mellitus was defined as plasma glucose ≥200 mg/dL, the use of medications for diabetes mellitus, or both. Body mass index was calculated as weight (kg) divided by the square of height (m). Public health nurses obtained information on smoking, drinking, and medical histories[13].
Follow-up Survey
To determine the cause of death after the 15-year follow-up, we used the National Vital Statistics Database of Japan with permission from the Management and Coordination Agency, Government of Japan. The underlying causes of death were coded according to the International Classification of Diseases, Ninth Revision (ICD-9) until the end of 1994, and according to the International Classification of Diseases, Tenth Revision (ICD-10) from the beginning of 1995. The details of the follow-up survey and the classification of causes of death in the present study are described elsewhere[7-19]. Permission to use the National Vital Statistics was obtained from the Management and Coordination Agency, of the national government of Japan. Because of the voluntary participation of community dwellers to the national survey, of which data was matched to the National Vital Statistics, and anonymous nature of the data for the analysis, informed consent was waived. Approval for the study was obtained from the institutional review board of Shiga University of Medical Science (No. 12-18, 2000).
Statistical Analysis
All data were expressed as mean ± standard deviation. Differences in each variable between participants with and those without APC were evaluated using the chi-square test or Fisher’s exact test for categorical variables and Student’s unpaired t-test for continuous variables. Event-free survival in patients with and those without APC was estimated by the Kaplan–Meier method, and statistical differences were evaluated by means of the log-rank test. Cox proportional hazard models were used to evaluate risk factor-adjusted associations of APC with each endpoint.
In Cox models with endpoints including all-cause deaths and CVD deaths, adjustment was made in Models 1, 2, and 3. Covariates in Model 1 were age and gender. Model 2 consisted of Model 1 plus the conventional risk factors of body mass index, current smoking, drinking habit (never, past, and current drinker categories, with “never drinker” as a reference), hypercholesterolemia, diabetes mellitus, systolic blood pressure, and serum creatinine. Model 3 consisted of Model 2 plus other ECG findings (high R-wave, ST depression, T-wave abnormality, ventricular premature complexes). Covariates included in these models were clinically associated with CVD, significantly related to CVD deaths by univariate analysis, and also included the factors that were associated with APC at a level of significance P < 0.05 at baseline.
Analyses of data were performed using SPSS, version 20 (SPSS Inc., Chicago, IL). All P-values were two-sided, and significance was defined as P < 0.05 for all analyses.
Results
Sixty-four participants (0.8%) had APC, as determined by the screening ECG. The associated variables at baseline and the participants’ clinical outcomes are summarized in Table 1. Age, systolic blood pressure, and certain ECG findings (ST depression and T-wave abnormality) were significantly different between participants with and those without APC.
Table 1. Baseline characteristics of the study group.
| APC |
|||
|---|---|---|---|
| No | Yes | ||
| (n = 7628) | (n = 64) | p | |
| Male, n (%) | 3159 (41.4%) | 32 (50.0%) | 0.165 |
| Age, years | 52.4 | 67.3 | <0.001 |
| BMI, kgm-2 | 22.9 | 22.0 | 0.022 |
| Systolic blood pressure (mmHg) | 135 | 148 | <0.001 |
| Diastolic blood pressure (mmHg) | 81 | 83 | 0.111 |
| Hypertension, n (%) | 1606 (21.1%) | 21 (32.8%) | 0.022 |
| Hypercholesterolemia, n (%) | 517 (6.8%) | 6 (9.4%) | 0.411 |
| Diabetes mellitus, n (%) | 362 (4.8%) | 4 (6.3%) | 0.59 |
| Current smoking, n (%) | 2185 (28.6%) | 16 (25.0%) | 0.521 |
| Drinking | |||
| Ex-drinker | 237 (3.1%) | 2 (3.1%) | 0.993 |
| Current drinker | 2156 (28.3%) | 16 (25%) | 0.564 |
| Laboratory tests | |||
| Total Cholesterol (mg/dL) | 204 | 203 | 0.892 |
| HDL Cholesterol (mg/dL) | 54 | 53 | 0.741 |
| Fasting blood glucose (mg/dL) | 103 | 106 | 0.218 |
| Creatinine (mg/dL) | 0.81 | 0.85 | 0.119 |
| Other ECG findings | |||
| QRS axis deviation, n (%) | 251 (3.3%) | 2 (3.1%) | 1 |
| High R, n (%) | 802 (10.5%) | 12 (18.8%) | 0.033 |
| ST depression, n (%) | 184 (2.4%) | 6 (9.4%) | 0.005 |
| T-wave abnormality, n (%) | 605 (7.9%) | 15 (23.4%) | <0.001 |
| Bundle branch block, n (%) | 559 (7.3%) | 4 (6.3%) | 1 |
| Rotation, n (%) | 3810 (50%) | 35 (54.7%) | 0.45 |
Abbreviations: APC, atrial premature complex; BMI, body mass index; HDL, high density lipoprotein; ECG, electrocardiogram. All values are expressed as the mean ± SD or as a number with the percentage of participants in parentheses.
The total follow-up time for the 7692 participants (men, 41.5%; mean age, 52.5 ± 13.7 years) in this study was 107,474 person-years (mean, 14.0 ± 2.9 years). During the follow-up, there were 1211 all-cause deaths and 338 CVD deaths, including 68 due to coronary artery disease, 73 due to heart failure, and 138 due to stroke. Table 2 represents the cumulative mortality, which indicated that participants with APC tended to have higher all-cause, CVD, heart failure or stroke mortality. Figure 2 shows Kaplan-Meier survival curves for CVD deaths in participants with and without APC. These curves indicate that participants with APC at baseline had a significantly lower survival rate during the follow-up period (P < 0.001 by log-rank test).
Table 2. Baseline ECG abnormalities and outcomes for the study group.
| APC |
|||
|---|---|---|---|
| No | Yes | ||
| (n = 7628) | (n = 64) | p | |
| Total death, n (%) | 1181 (15.5%) | 30 (46.9%) | <0.001 |
| CVD death, n (%) | 326 (4.3%) | 12 (18.8%) | <0.001 |
| CHD death, n (%) | 67 (0.9%) | 1 (1.6%) | 0.388 |
| HF death, n (%) | 69 (0.9%) | 4 (6.3%) | <0.001 |
| Stroke death, n (%) | 133 (1.7%) | 5 (7.8%) | <0.001 |
Abbreviations: ECG, electrocardiogram; APC, atrial premature complex; CVD, cardiovascular disease; CHD, coronary heart disease; HF, heart failure. All values are expressed as a number with the percentage of participants in parentheses.
The statistical differences were evaluated using a log-rank test.
Figure 2. Kaplan–Meier estimates of cumulative hazard for cardiovascular disease for participants with and those without APC.
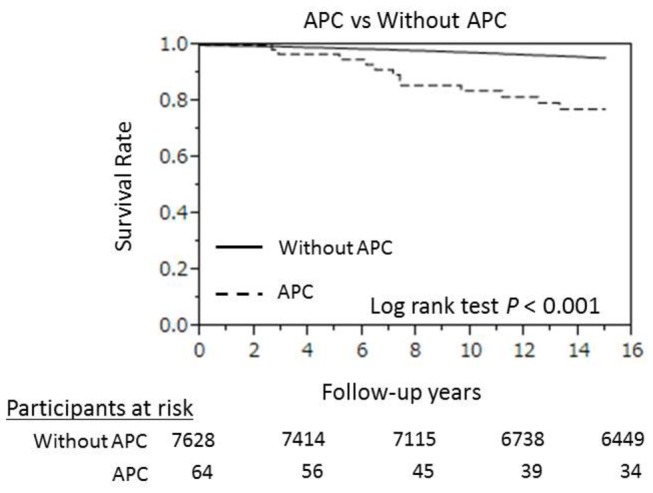
APC, atrial premature complexes.
Cox regression analysis (Table 3) revealed that the presence of APC was an independent predictor for all-cause death (HR: 1.55, 95% CI: 1.07–2.24, P = 0.020) and CVD death (HR: 2.03, 95% CI: 1.12–3.66, P = 0.019).
Table 3. Hazard ratios for each outcome by ECG abnormality status at baseline.
| APC | p value | |
|---|---|---|
| HR (95% CI) | ||
| All causes mortality | ||
| Unadjusted | 3.98 (2.77-5.71) | <0.001 |
| Model 1 | 1.50 (1.04-2.16) | 0.03 |
| Model 2 | 1.60 (1.11-2.31) | 0.012 |
| Model 3 | 1.55 (1.07-2.24) | 0.020 |
| Cardiovascular death | ||
| Unadjusted | 5.79 (3.25-10.3) | <0.001 |
| Model 1 | 2.06 (1.15-3.68) | 0.015 |
| Model 2 | 2.11 (1.17-3.81) | 0.013 |
| Model 3 | 2.03 (1.12-3.66) | 0.019 |
Abbreviation: ECG, electrocardiogram; APC, atrial premature complex; HR, hazard ratio; CI, Confidence Interval.
Multivariate-Adjusted for Model 1 (age and gender), Model 2 (age, gender, body mass index, smoking habit, drinking habit, hypercholesterolemia, diabetes mellitus, systolic blood pressure, serum creatinine) and Model 3 (Model 2 + other ECG findings).
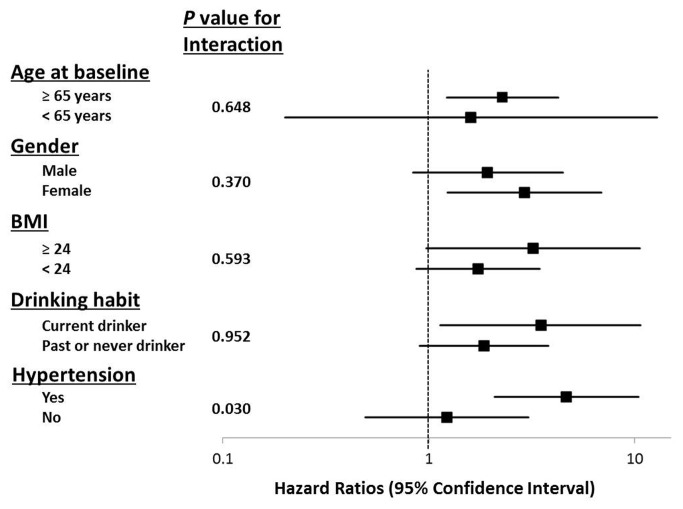
As shown in Figure 3, the impact of APC on CVD death was more evident in participants with hypertension. The probability value for an interaction between APC and hypertension on CVD death was 0.03. Indeed, the adjusted hazard ratios of APC for all-cause death (HR: 3.19, 95% CI: 1.87–5.45, P < 0.001) and CVD death (HR: 4.66, 95% CI: 2.09–10.4, P < 0.001) were statistically significant in the hypertensive participants. Further adjustment of anti-hypertensive medication did not alter the results (data not shown). Independent analysis of other major subgroups such as the elderly (≥65 years of age), women, obese individuals (body mass index ≥ 24 kg/m2) and current drinkers did not yield significant results (Figure 3).
Figure 3. Adjusted hazard ratios of atrial premature complexes for cardiovascular disease deaths in various subgroups.
Multivariate-adjusted Model 3 (age, gender, body mass index, smoking habit, drinking habit, hypercholesterolemia, diabetes mellitus, systolic blood pressure, serum creatinine, and other ECG findings).
BMI, body mass index.
Discussion
The major finding of this prospective cohort study of a large sample of healthy Japanese individuals was that at least one beat of APC, recorded through the screening ECG, was associated with an increased risk of CVD mortality in healthy Japanese participants. Although APC was not frequently observed in our study (less than 1%), this long-term follow-up study clarified its potential hazards. Of note, this adverse effect of APC was particularly prominent in hypertensive participants.
The clinical significance of APC has been investigated for decades. Recent studies of the 24-hour ambulatory ECG have demonstrated that excessive APC are associated with a higher risk for the development of atrial fibrillation and CVD mortality[4,6,20]. Our findings extend the prognostic significance of APC to healthy participants who have premature complexes recorded on a routine 12-lead ECG. In this cohort, with a follow-up of more than 100,000 person-years (mean, 14.0 ± 2.9 years), the finding of APC on routine ECG was associated with an increase in CVD mortality, particularly due to heart failure and stroke.
The mechanisms by which APC may increase the risk of heart failure are not clear, but several possibilities exist. First, APC may be an early manifestation of underlying heart disease that elevates left ventricular (LV) filling pressure. Early diastolic dysfunction may lead to elevated LV filling without clinical manifestations, with the exception of subtle left atrial remodeling that may be the cause of frequent APC[21]. A previous study conducted in the general community using Doppler echocardiography reported that although only approximately 5% of the population had systolic dysfunction, as many as approximately 30% had diastolic dysfunction, regardless of its severity, without any apparent heart failure[22]. In addition, some reports demonstrated that excessive APC were observed in participants with higher levels of N-terminal prohormone B-type natriuretic peptide[2,4]. These previous reports suggest that many general participants may have subclinical heart failure status, and APC are likely to become apparent with increased filling pressure. The adverse impact of APC on long-term outcome was apparent in participants with hypertension, which is associated with an increased LV filling pressure and diastolic dysfunction[23].
The other likely explanation is that APC triggers atrial fibrillation (AF) or is a forerunner of AF, resulting in the exacerbation of heart failure. Recent studies using 24-hour ECG monitoring demonstrated that frequent APC were correlated with a new occurrence of AF[4,6], while studies conducted in patients with acute stroke revealed that frequent APC predicted paroxysmal AF[20,24]. These findings suggest that APC could be a surrogate marker or a prodromal stage of AF, which is one of the independent prognostic factors in participants with heart failure. Furthermore, current concepts support the view that this paroxysmal or subclinical AF is also associated with an increased risk of acute stroke[25]. In our study, the presence of APC was associated with an increased risk of CVD mortality, particularly due to heart failure and stroke, reinforcing the possibility of an association between the presence of APC and the occurrence of AF, eventually leading to cardioembolic strokes[26].
Although in the present study, we have shown that CVD deaths occur twice as frequently in participants with APC than in those without APC, a previous large-scale study from the atherosclerosis risk in communities (ARIC) study reported conflicting results[3]. The latter study, however, focused only on sudden cardiac death or coronary heart disease, whereas the incidence of all CVD deaths, including heart failure or stroke—which are considered to be the most important outcomes related to APC—was not evaluated. These differences regarding endpoints might have reduced the prognostic impact of APC in that study.
It remains controversial whether an ECG examination should be performed in asymptomatic individuals. Our findings regarding APC are in concordance with western guidelines, which recommend the use of ECG screening in participants who have a high risk for CVD, such as patients with hypertension[27,28]. Screening for APC and other electrocardiographic abnormalities may be of assistance in performing further risk stratification in these patients; patients in a higher risk category may benefit from aggressive lifestyle modification or cardiovascular investigation. On the other hand, our results also demonstrated few associations of APC and CVD mortality in participants with lower risk for underlying heart diseases, especially in participants without hypertension, which may justify not performing ECG screening for apparently healthy population.
Limitations
Our study has several limitations. First, although the participants in our cohort were limited to Japanese populations only, previous studies have demonstrated that the prognostic values of other ECG findings, proven in the United States or Europe, were also applicable to Japanese populations[8,12,17,29]. Asians reportedly have a different risk profile from western populations, with a significantly better overall cardiovascular prognosis[30,31], but a higher incidence of stroke than of coronary artery disease[32]. As shown in this study, the importance of APC as an independent predictor of stroke is beginning to be understood, and it is meaningful to evaluate the association of APC and adverse CVD outcomes, including stroke, in such a Japanese population. Second, sub-classifications of stroke events were not available. Although the etiology of stroke associated with premature complexes is likely to include the increased risk of cardioembolism, further studies are needed to confirm this. Last, we used a single ECG at baseline. Single biological measurements are known to be subject to variability. Therefore, the sensitivity of a single 12-lead ECG for the detection of premature complexes should be lower than that for more sensitive strategies, including 2-minute rhythm strips or a 24-hour ambulatory ECG. However, the presence of premature complexes on a single 12-lead ECG recorded in only 6 seconds might indicate that participants with such APC were likely to have frequent ectopic beats as a whole.
Conclusions
APC recorded during the screening ECG serve as an independent predictor of CVD deaths in a Japanese community-dwelling population. This tendency seemed to be most apparent in hypertensive participants, in whom the presence of APC was a predictor of CVD death, particularly due to heart failure and stroke.
Acknowledgments
The authors would like to thank the members of the NIPPON DATA80/90 Research Group, who are listed below.
The members of the NIPPON DATA80/90 Research Group are: Chairperson: Hirotsugu Ueshima (Department of Health Science, Shiga University of Medical Science, Otsu, Shiga).
Co-Chairperson: Akira Okayama (The First Institute for Health Promotion and Health Care, Japan Anti-Tuberculosis Association, Tokyo) for the NIPPON DATA80, Tomonori Okamura (Department of Preventive Medicine and Public Health, Keio University, Tokyo) for the NIPPON DATA90.
Research members: Shigeyuki Saitoh (Department of 2nd Internal Medicine, Sapporo Medical University, Sapporo, Hokkaido), Kiyomi Sakata (Department of Hygiene and Preventive Medicine, Iwate Medical University, Morioka, Iwate), Atsushi Hozawa (Department of Public Health, Yamagata University Graduate School of Medicine, Yamagata), Takehito Hayakawa (Department of Hygiene and Preventive Medicine, Fukushima Medical University, Fukushima), Yosikazu Nakamura (Department of Public Health, Jichi Medical University, Shimotsuke, Tochigi), Yasuhiro Matsumura(Faculty of Healthcare, Kiryu University, Midori City, Gunma), Nobuo Nishi (Project for the National Health and Nutrition Survey, National Institute of Health and Nutrition, Tokyo), Nagako Okuda (The First Institute for Health Promotion and Health Care, Japan Anti-Tuberculosis Association, Tokyo), Toru Izumi (Faculty of Medicine, Kitasato University, Sagamihara, Kanagawa), Toshiyuki Ojima (Department of Community Health and Preventive Medicine, Hamamatsu University School of Medicine, Hamamatsu, Shizuoka), Koji Tamakoshi (Department of Public Health and Health Information Dynamics, Nagoya University Graduate School of Medicine, Nagoya, Aichi), Hideaki Nakagawa (Department of Epidemiology and Public Health, Kanazawa Medical University, Kanazawa, Ishikawa), Katsuyuki Miura, Yoshikuni Kita, Aya Kadota (Department of Health Science, Shiga University of Medical Science, Otsu, Shiga), Takayoshi Ohkubo (Department of Hygiene and Public Health, Teikyo University School of Medicine), Yasuyuki Nakamura (Cardiovascular Epidemiology, Kyoto Women's University, Kyoto), Katsushi Yoshita (Osaka City University Graduate School of human life science, Osaka), Kazunori Kodama, Fumiyoshi Kasagi (Radiation Effects Research Foundation, Hiroshima), and Yutaka Kiyohara (Department of Environmental Medicine, Kyushu University, Fukuoka).
All authors take responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation.
Funding Statement
This study was supported by a grant-in-aid of the Ministry of Health, Labor, and Welfare under the auspices of the Japanese Association for Cerebro-Cardiovascular Disease Control; a Research Grant for Cardiovascular Diseases (7A-2) from the Ministry of Health, Labor, and Welfare; and a Health and Labor Sciences Research Grant, Japan (Comprehensive Research on Aging and Health [H11-Chouju-046, H14-Chouju-003, H17-Chouju-012, H19-Chouju-Ippan-014] and Comprehensive Research on Life Style–Related Diseases Including Cardiovascular Diseases and Diabetes Mellitus [H22-Jyunkankitou-Seisyu-Sitei-017, H25-Jyunkankitou-Seisyu-Sitei-022]). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. World Health Organization (2008) The Global Burden of Disease: 2004 Update. Geneva, World Health Organization. [Google Scholar]
- 2. Conen D, Adam M, Roche F, Barthelemy JC, Felber Dietrich D et al. (2012) Premature Atrial Contractions in the General Population: Frequency and Risk Factors. Circulation 126: 2302-2308. doi: 10.1161/CIRCULATIONAHA.112.112300. PubMed: 23048073. [DOI] [PubMed] [Google Scholar]
- 3. Cheriyath P, He F, Peters I, Li X, Alagona P Jr. et al. (2011) Relation of atrial and/or ventricular premature complexes on a two-minute rhythm strip to the risk of sudden cardiac death (the Atherosclerosis Risk in Communities [ARIC] study). Am J Cardiol 107: 151-155. doi: 10.1016/j.amjcard.2010.09.002. PubMed: 21211594. [DOI] [PubMed] [Google Scholar]
- 4. Binici Z, Intzilakis T, Nielsen OW, Køber L, Sajadieh A (2010) Excessive supraventricular ectopic activity and increased risk of atrial fibrillation and stroke. Circulation 121: 1904-1911. doi: 10.1161/CIRCULATIONAHA.109.874982. PubMed: 20404258. [DOI] [PubMed] [Google Scholar]
- 5. Engström G, Hedblad B, Juul-Möller S, Tydén P, Janzon L (2000) Cardiac arrhythmias and stroke: increased risk in men with high frequency of atrial ectopic beats. Stroke 31: 2925-2929. doi: 10.1161/01.STR.31.12.2925. PubMed: 11108750. [DOI] [PubMed] [Google Scholar]
- 6. Chong BH, Pong V, Lam KF, Liu S, Zuo ML et al. (2012) Frequent premature atrial complexes predict new occurrence of atrial fibrillation and adverse cardiovascular events. Europace 14: 942-947. doi: 10.1093/europace/eur389. PubMed: 22183750. [DOI] [PubMed] [Google Scholar]
- 7. Nakamura Y, Okamura T, Higashiyama A, Watanabe M, Kadota A et al. (2012) Prognostic values of clockwise and counterclockwise rotation for cardiovascular mortality in Japanese subjects: a 24-year follow-up of the National Integrated Project for Prospective Observation of Noncommunicable Disease and Its Trends in the Aged, 1980-2004 (NIPPON DATA80). Circulation 125: 1226-1233. doi: 10.1161/CIRCULATIONAHA.111.070045. PubMed: 22308300. [DOI] [PubMed] [Google Scholar]
- 8. Rumana N, Turin TC, Miura K, Nakamura Y, Kita Y et al. (2011) Prognostic value of ST-T abnormalities and left high R waves with cardiovascular mortality in Japanese (24-year follow-up of NIPPON DATA80). Am J Cardiol 107: 1718-1724. doi: 10.1016/j.amjcard.2011.02.335. PubMed: 21497783. [DOI] [PubMed] [Google Scholar]
- 9. Nakamura K, Okamura T, Hayakawa T, Kadowaki T, Kita Y, et al. (2006) Electrocardiogram screening for left high R-wave predicts cardiovascular death in a Japanese community-based population: NIPPON DATA90. Hypertens Res 29: 353-360. [DOI] [PubMed] [Google Scholar]
- 10. Kadota A, Miura K, Okamura T, Hozawa A, Murakami Y, et al. (2011) Relationship of moderate metabolic risk factor clustering to cardiovascular disease mortality in non-lean Japanese: a 15-year follow-up of NIPPON DATA90. Atherosclerosis 215: 209-213. [DOI] [PubMed] [Google Scholar]
- 11. Higashiyama A, Watanabe M, Kokubo Y, Ono Y, Okayama A, et al. (2010) Relationships between protein intake and renal function in a Japanese general population: NIPPON DATA90. J Epidemiol 20 Suppl 3: S537-543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Horibe H, Kasagi F, Kagaya M, Matsutani Y, Okayama A, et al. (2005) A nineteen-year cohort study on the relationship of electrocardiographic findings to all cause mortality among subjects in the national survey on circulatory disorders, NIPPON DATA80. J Epidemiol 15: 125-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ueshima H, Choudhury SR, Okayama A, Hayakawa T, Kita Y, et al. (2004) Cigarette smoking as a risk factor for stroke death in Japan: NIPPON DATA80. Stroke 35: 1836-1841. [DOI] [PubMed] [Google Scholar]
- 14. Okayama A, Kadowaki T, Okamura T, Hayakawa T, Ueshima H (2006) Age-specific effects of systolic and diastolic blood pressures on mortality due to cardiovascular diseases among Japanese men (NIPPON DATA80). J Hypertens 24: 459-462. Available online at: doi:10.1097/01.hjh.0000209981.43983.cf. PubMed: 16467648. [DOI] [PubMed] [Google Scholar]
- 15. Okamura T, Tanaka H, Miyamatsu N, Hayakawa T, Kadowaki T et al. (2007) The relationship between serum total cholesterol and all-cause or cause-specific mortality in a 17.3-year study of a Japanese cohort. Atherosclerosis 190: 216-223. doi: 10.1016/j.atherosclerosis.2006.01.024. PubMed: 16529754. [DOI] [PubMed] [Google Scholar]
- 16. Kadowaki S, Okamura T, Hozawa A, Kadowaki T, Kadota A, et al. (2008) Relationship of elevated casual blood glucose level with coronary heart disease, cardiovascular disease and all-cause mortality in a representative sample of the Japanese population. NIPPON DATA80. Diabetologia 51: 575-582. [DOI] [PubMed] [Google Scholar]
- 17. Ohsawa M, Okayama A, Okamura T, Itai K, Nakamura M, et al. (2007) Mortality risk attributable to atrial fibrillation in middle-aged and elderly people in the Japanese general population: nineteen-year follow-up in NIPPON DATA80. Circ J 71: 814-819. [DOI] [PubMed] [Google Scholar]
- 18. Okamura T, Hayakawa T, Kadowaki T, Kita Y, Okayama A et al. (2004) Resting heart rate and cause-specific death in a 16.5-year cohort study of the Japanese general population. Am Heart J 147: 1024-1032. doi: 10.1016/j.ahj.2003.12.020. PubMed: 15199351. [DOI] [PubMed] [Google Scholar]
- 19. Okamura T, Hayakawa T, Kadowaki T, Kita Y, Okayama A et al. (2006) The inverse relationship between serum high-density lipoprotein cholesterol level and all-cause mortality in a 9.6-year follow-up study in the Japanese general population. Atherosclerosis 184: 143-150. doi: 10.1016/j.atherosclerosis.2005.03.042. PubMed: 15913635. [DOI] [PubMed] [Google Scholar]
- 20. Wallmann D, Tüller D, Wustmann K, Meier P, Isenegger J et al. (2007) Frequent atrial premature beats predict paroxysmal atrial fibrillation in stroke patients: an opportunity for a new diagnostic strategy. Stroke 38: 2292-2294. doi: 10.1161/STROKEAHA.107.485110. PubMed: 17585079. [DOI] [PubMed] [Google Scholar]
- 21. Abhayaratna WP, Seward JB, Appleton CP, Douglas PS, Oh JK et al. (2006) Left atrial size: physiologic determinants and clinical applications. J Am Coll Cardiol 47: 2357-2363. doi: 10.1016/j.jacc.2006.02.048. PubMed: 16781359. [DOI] [PubMed] [Google Scholar]
- 22. Redfield MM, Jacobsen SJ, Burnett JJ, Mahoney DW, Bailey KR et al. (2003) Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA 289: 194-202. doi: 10.1001/jama.289.2.194. PubMed: 12517230. [DOI] [PubMed] [Google Scholar]
- 23. Diamond JA, Phillips RA (2005) Hypertensive heart disease. Hypertens Res 28: 191-202. doi: 10.1291/hypres.28.191. PubMed: 16097361. [DOI] [PubMed] [Google Scholar]
- 24. Wallmann D, Tüller D, Kucher N, Fuhrer J, Arnold M et al. (2003) Frequent atrial premature contractions as a surrogate marker for paroxysmal atrial fibrillation in patients with acute ischaemic stroke. Heart 89: 1247-1248. doi: 10.1136/heart.89.10.1247. PubMed: 12975433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Healey JS, Connolly SJ, Gold MR, Israel CW, Van Gelder IC et al. (2012) Subclinical atrial fibrillation and the risk of stroke. N Engl J Med 366: 120-129. doi: 10.1056/NEJMoa1105575. PubMed: 22236222. [DOI] [PubMed] [Google Scholar]
- 26. Wolf PA, Abbott RD, Kannel WB (1991) Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke 22: 983-988. doi: 10.1161/01.STR.22.8.983. PubMed: 1866765. [DOI] [PubMed] [Google Scholar]
- 27. Greenland P, Alpert JS, Beller GA, Benjamin EJ, Budoff MJ et al. (2010) 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 122: e584-e636. doi: 10.1161/CIR.0b013e3182051b4c. PubMed: 21098428. [DOI] [PubMed] [Google Scholar]
- 28. U.S. Preventive Services Task Force (2004) Screening for coronary heart disease: recommendation statement. Ann Intern Med 140: 569-572. [DOI] [PubMed] [Google Scholar]
- 29. Higashiyama A, Hozawa A, Murakami Y, Okamura T, Watanabe M et al. (2009) Prognostic value of q wave for cardiovascular death in a 19-year prospective study of the Japanese general population. J Atheroscler Thromb 16: 40-50. doi: 10.5551/jat.E606. PubMed: 19261999. [DOI] [PubMed] [Google Scholar]
- 30. Santos-Gallego CG, Badimón JJ (2012) Risk factors: Ethnicity and cardiovascular risk-are all men created equal? Nat Rev Cardiol 9: 10-12. doi: 10.1038/nchembio.1143. PubMed: 22105672. [DOI] [PubMed] [Google Scholar]
- 31. Kohsaka S, Kimura T, Goto M, Lee VV, Elayda M et al. (2010) Difference in patient profiles and outcomes in Japanese versus American patients undergoing coronary revascularization (collaborative study by CREDO-Kyoto and the Texas Heart Institute Research Database). Am J Cardiol 105: 1698-1704. doi: 10.1016/j.amjcard.2010.01.349. PubMed: 20538117. [DOI] [PubMed] [Google Scholar]
- 32. Ueshima H, Sekikawa A, Miura K, Turin TC, Takashima N et al. (2008) Cardiovascular disease and risk factors in Asia: a selected review. Circulation 118: 2702-2709. doi: 10.1161/CIRCULATIONAHA.108.790048. PubMed: 19106393. [DOI] [PMC free article] [PubMed] [Google Scholar]