Abstract
Allergic skin inflammation such as atopic dermatitis (AD) is characterized by edema and infiltration with various inflammatory cells such as mast cells, basophils, eosinophils and T cells. Thymic stromal lymphopoietin (TSLP) is produced mainly by epidermal keratinocytes, as well as dermal fibroblasts and mast cells in the skin lesions of AD. Omega-3 polyunsaturated fatty acids in fish oil can reduce inflammation in allergic patients. Fermentation has a tremendous capacity to transform chemical structures. The antiinflammatory effects of fish oil have been described in many diseases, but the beneficial effects by which fermented olive flounder oil (FOF) modulates the allergic response is poorly understood. In this study, we produced FOF and tested its ability to suppress the various allergic inflammatory responses. The ability of FOF to modulate the immune system was investigated using a mouse model of AD. The FOF-treated group showed significantly decreased immunoglobulin E (IgE) and histamine in serum. Also, the increased TSLP expression was significantly inhibited in the FOF group; the FOF-treated group was not appreciably different from the hydrocort cream treatment group. In addition, FOF treatment resulted in a smaller spleen size with reduced the thickness and length compared to the induction group. Splenocytes from mice treated with FOF produced significantly less IFN-γ, IL-4, T-box transcription factor (T-bet) and GATA binding protein 3 (GATA3) expression compared with the induction group. These results suggest that FOF may be effective in treating the allergic symptoms of AD. 5.
Keywords: Atopic dermatitis, Fermented olive flounder oil, Thymic stromal lymphopoietin, T-box transcription factor, GATA binding protein 3
INTRODUCTION
Atopic dermatitis (AD), a chronic inflammatory skin disease associated with skin hyper-reactivity, affects approximately 10~20% of children and 1~3% of adults worldwide (Leung et al., 2004). This systemic disorder is caused by skin barrier dysfunction and severe skin dehydration. The skin lesions of AD are generally characterized by infiltration with various inflammatory cells such as mast cells, basophils, eosinophils and T cells (Li et al., 2010; Li et al., 2005; Nystad et al., 2005; Wahlgren, 1999).
Activated mast cells release a variety of inflammatory mediators following cross-linking of immunoglobulin E (IgE)-receptor. Of these mediators, histamine is a violent inducer of itching and histamine is a characteristic major mediator in mast cell storage granules and directly triggers type I allergic responses (Kawakami et al., 2009; Kitamura and Ito, 2005; Leitges et al., 2002; Schwartz, 2004; Stone et al., 2010).
Thymic stromal lymphopoietin (TSLP) is produced mainly by epidermal keratinocytes, as well as dermal fibroblasts and mast cells in the skin lesions of acute and chronic AD (Soumelis et al., 2002). It can initiate and regulate the aller-gic inflammation reaction (Al-Shami et al., 2005; Bogiatzi et al., 2007). Moreover, TSLP can stimulate naive T cell to express pro-inflammatory cytokines (IL-4, -5 and -13) (Ebner et al., 2007).
T cells play a central role in cell-mediated immunity. The differentiation of naive T-helper (Th) precursor cells towards Th1 or Th2 effector cells is regulated by the transcription factors T-box expressed in T-cells (T-bet) and GATA-binding protein-3 (GATA-3) (Ouyang et al., 1998). Chronic AD is characterized by Th1-mediated immune responses. Th1 cells express large amounts of interferon-gamma (IFN-γ) and regulate the development of Th2 cells (Glimcher and Murphy, 2000). Atopic dermatitis is associated with increased Th2 cells in acute phase (Elson et al., 1998). The signature Th2 cytokine is interleukin (IL)-4 which can suppress the generation of Th1 cells from nave T cells in AD (Romagnani, 2000).
Several previous studies have examined the anti-allergic effects of fish oil. Among the fish, olive flounder (Paralichthys olivaceus) is a pleuronectiforms contains high concentration of omega-3 polyunsaturated fatty acid (n-3 PUFAs), such as eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) (Ginsberg and Toal, 2009; Seki et al., 2010). Fish oil intake can reduce sensitization to allergens, alleviate the severity of AD, eczema, and asthma, and down-regulate the expression of IL-1, -4, and -13 and IFN-γ in serum (Krauss-Etschmann et al., 2008; Kremmyda et al., 2011).
Recent studies have investigated the anti-allergic effects of fermented food products such as yogurt, cheese, and soybean paste on a variety of allergic reactions, diabetes and some cancers (Isobe et al., 2008; Kwon et al., 2010; Uchida et al., 2010). Fermentation can transmute the chemical structure of some constituents to create new substances. The beneficial effects of fish oil have been described in many diseases, but the mechanism by which fermented olive flounder oil (FOF) modulates the immune system and the allergic response is poorly understood. In this study, we produced FOF and tested its ability to suppress the various allergic inflammatory responses. The ability of FOF to modulate the immune system was investigated using a mouse model of AD.
MATERIALS AND METHODS
Experimental animals. BALB/c mice (female, 7-weeksold) were purchased from Orient Bio (Korea) and were maintained for 1 week before the start of any experiments. The mice were housed in the animal facility of Jeju National University under controlled temperature (23 ± 1℃), humidity (60 ± 10%), and light (lights on from 08:00 to 20:00 hours) and under pathogen-free conditions. All animal experiments were approved by the Animal Care and Use Committee at Jeju National University.
Fermentation of olive flounder oil. FOF was provided by Fermentec Inc. (Jeju, Korea). Pulverized fish byproduct was mixed with water, raw sugar, Lactobacillus plantarum and Saccharomyces cerevisiae under anaerobic fermentation conditions. After 15 days, hexane was added to the fermented fish liquid. FOF was separated from the hexane extract by rotary evaporation.
DNCB application to induce experimental AD. Mice were divided into five groups (normal, induction, positive control and FOF; n = 8 mice per group). Mice were painted with 100 μl of 1% DNCB or vehicle on their abdomen as the first sensitization (day-7). On day 0, the mice were sensitized with 100 μl of 0.5% DNCB on their ears and were then sensitized with 100 μl of 0.5% DNCB every other day for up to 31 days. From day 12 until the completion of the experiment, mice were painted with hydrocort cream (Green Cross, Korea) containing 2 mg/g hydrocortisone valerate and FOF (2 and 10%) on their ears every other day. The mice were sacrificed on day 32.
Macroscopic edema and histological evaluation. In the experimental AD mouse model, DNCB stimulation elicited ear edema and ear thickness was measured using a Digital Thickness Gauge (Mitutoyo). Ear tissues were fixed with 10% formalin and embedded in paraffin. Paraffin sections (3 μm each) were stained with H&E.
Immunohistochemistry (IHC) assay for detection of TSLP in ear tissue. To analyze the expression of TSLP in each ear, paraffin sections (3 μm, each) of ear tissue were prepared. IHC was performed with rabbit anti-TSLP (Novus Biologicals) and a rabbit-specific HRP/DAB detection IHC kit (Abcam) according to the manufacturer’s instructions.
Western blot for detection of TSLP in ear tissue. Total protein was isolated from ear tissue using lysis buffer and the protein concentration of each sample was quantified by the bradford assay. The proteins were electroblotted onto a polyvinylidene difluoride (PVDF) membrane using an iBlot gel transfer device (Invitrogen). The membrane was incubation with rabbit anti-TSLP (Novus Biologicals). After washing, the membrane was incubated with horseradish peroxidase (HRP)-conjugated anti-rabbit IgG. The blot was visualized with a western blot detection system (iNtRON Biotechnology) according to the manufacturer’s instructions.
Splenocyte culture in BALB/c mouse. Mice from each group were sacrificed by cervical dislocation and their spleens were removed aseptically. To obtain single-cell suspensions, red blood cells were removed using red blood cell lysis buffer. After washing, splenocytes (1.0 × 106 cells/ml) were incubated in the presence or absence of anti-CD3 (1 μg/ml) and anti-CD28 (0.5 μg/ml) (eBioscience) for 3 days. Following incubation, the supernatants were collected to determine levels of IL-4 and IFN-γ and the cells were immediately analyzed by flow cytometric analysis (BD Biosciences).
ELISA. IgE (Biolegend, San Diego, CA) and histamine (Labor Diagnostika Nord, Nordhorn, Germany) in mouse serum and IL-4 and IFN-γ (R&D Systems, St. Louis, MO) in the supernatant of cultured cells were measured using ELISA kits according to the manufacturer’s instructions.
Flow cytometric analysis (FACS). To analyze Foxp3 expression, cells were permeabilized with the T-bet/GATA3 fixation/permeabilization kit (BD Biosciences), and stained with anti-T-bet-Alexa Fluor® 488 and anti-GATA3-PE (BD Biosciences) according to the manufacturer’s instructions. Briefly, to block mouse Fc receptors, the cell suspension was incubated with CD16/CD32 (BD Biosciences) for 15 min, followed by incubation in fixation/permeabilization buffer for 20 min, and anti-T-bet-Alexa Fluor® 488 and anti-GATA3-PE for 30 min.
Statistical analysis. Quantity One version 4.2.1 and Image-Pro plus version 4.5 software were used to transform images into numerical values. Student’s t-test and twoway analysis of variance were used to determine the statistical significance of differences between experimental and induction groups. Data are shown as mean ± standard deviation.
RESULTS
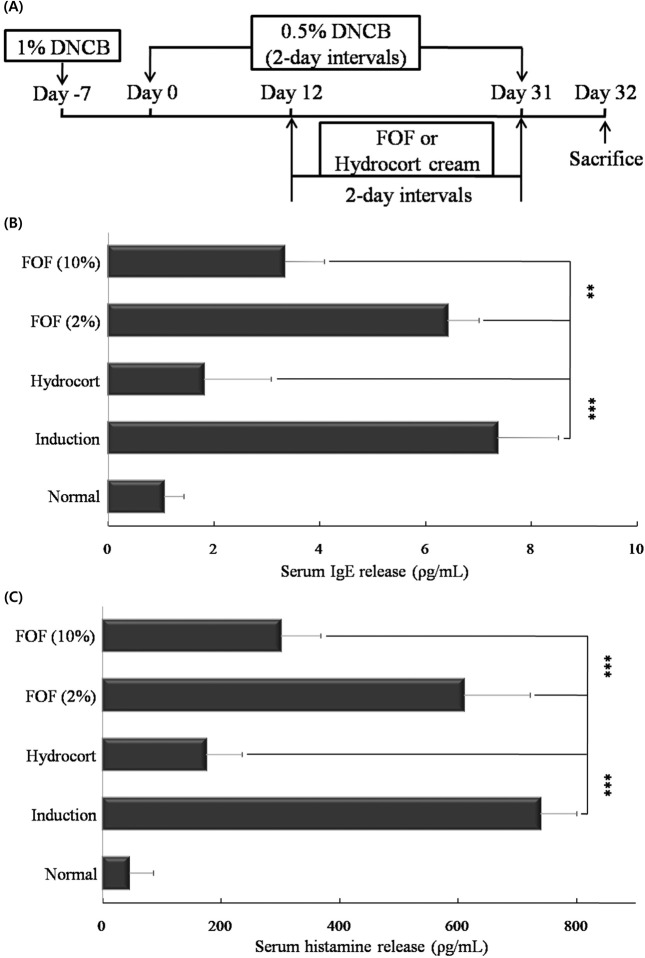
FOF suppresses the expression of serum IgE and histamine. To induce experimental AD, mice were stimulated an initial sensitization with 1% dinitrochlorobenzene (DNCB) on the abdomen, followed by sensitization with 0.5% DNCB on their ears every other day for up to 31 days. Starting on day 12, the mice were painted with hydrocort cream and FOF (2 and 10%) on their ears every other day. On day 32, all mice were sacrificed (Fig. 1A).
Fig. 1. FOF suppresses the expression of serum IgE and histamine. (A) Mice were painted with 1% DNCB or vehicle on their abdomen as the first sensitization (day-7). On day 0, the mice were sensitized with 0.5% DNCB on their ears and were then sensitized with 0.5% DNCB every other day for up to 31 days. From day 12 until the completion of the experiment, mice were painted with hydrocort cream and FOF (2 and 10%) on their ears every other day. The mice were sacrificed on day 32. (B) After sacrifice, the IgE and histamine in mouse serum was measured by ELISA. Data are representative of 8 mice per group. Values are mean ± S.D. (n = 8 mice per group). **, *** compared to mice stimulated with DNCB alone (induction group). **P< 0.005; *** P< 0.001.
IgE is an crucial therapeutic target for AD as it is the major mechanism for activating mast cells to release histamine (Levin et al., 2006). Therefore, we tested serum IgE and histamine levels in mice with dermatitis by Enzymelinked immunosorbent assay (ELISA). The 10% FOFtreated group showed significantly decreased IgE (P < 0.005) and histamine (P < 0.001) levels compared with the induction group. In addition, 10% FOF treatment had a stronger inhibitory effect than 2% FOF on allergic symptoms (Fig. 1B and C).
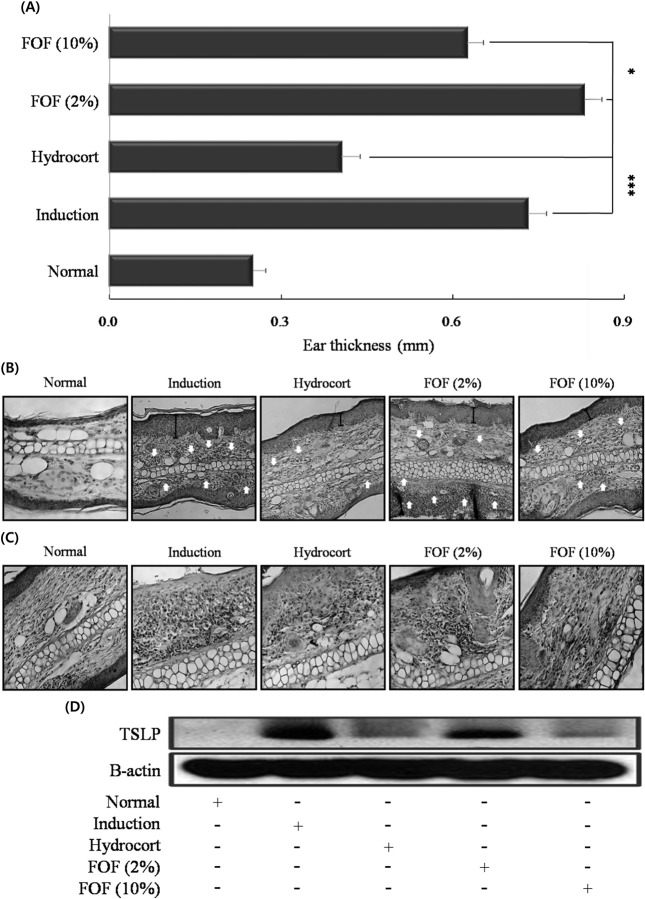
Histological features of ear tissue and TSLP hyperproduction in DNCB-stimulated mice. The skin lesions of patients with AD are characterized by infiltration of various inflammatory cells such as granulocytes, and T cells (de Vries et al., 1997). Therefore, we measured whether FOF treatment alleviates inflammatory cell infiltration in the ears of mice with experimental AD. Skin swelling as a measure of AD progression was also measured. 10% FOF treatment (P < 0.05) reduced ear thickness (Fig. 2A). The effect of FOF treatment on infiltration of inflammatory cells into the ear tissue was monitored by hematoxylin and eosin (H&E) staining. The 10% FOF-treated group showed significantly reduced epidermal thickness and inflammatory cell infiltration compared with the induction group (Fig. 2B).
Fig. 2. Histological features of ear tissue and TSLP hyperproduction in DNCB-stimulated mice. BALB/c mice were sensitized with DNCB at 2-day intervals for up to 31 days, painted with hydrocort cream and FOF (2 and 10%) every other day, as described in Figure legend 1. Ear thickness was measured on day 31. (B) Paraffin sections of ear tissues were stained with hematoxylin and eosin (H&E, × 200), and (C) processed for TSLP immunohistochemistry (IHC, × 200). (E) TSLP was measured in ear tissues by western blot. Data are representative of 8 mice per group. Values are mean ± S.D. (n = 8 mice per group). *P< 0.05 and ***P< 0.001 compared to mice stimulated with DNCB alone (induction group).
TSLP produced by epidermal keratinocytes, dermal fibro-blasts and mast cells can regulate allergic inflammation reactions (Bogiatzi et al., 2007; Soumelis et al., 2002). Therefore, we measured the expression of TSLP in ear tissue of AD mice models using IHC and western blot. The elevated TSLP expression was significantly inhibited in the 10% FOF group; the 10% FOF-treated group was not appreciably different from the hydrocort cream treatment group (Fig. 2C and D). In addition, 10% FOF treatment showed stronger inhibitory effects than 2% FOF on cutaneous edema and TSLP hyperproduction.
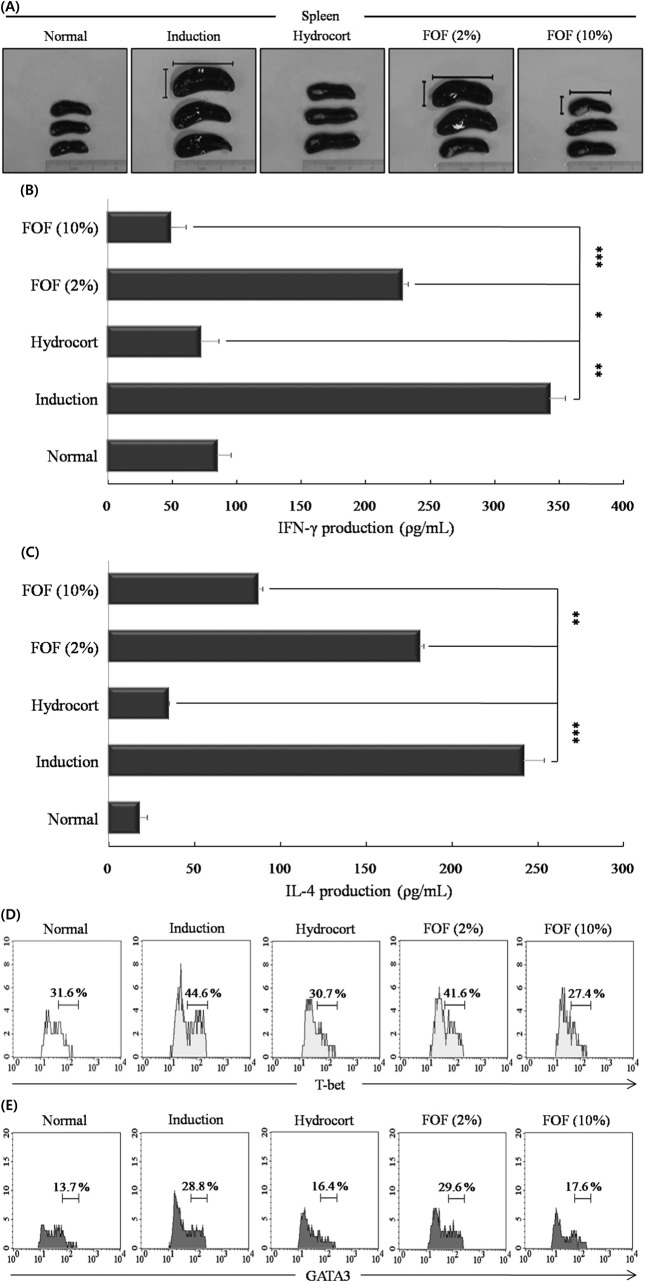
FOF decreases the function of mature Th1/2 cells in experimental AD models. The spleen plays crucial roles in the active immune system through the cell-mediated pathway, especially through the activity of mature T and B cells (Debes et al., 2004). Therefore, we observed the morphologic features of the spleen. Mice in induction group showed a highly enlarged spleen. 10% FOF treatment resulted in a smaller spleen size with reduced the thickness and length compared to the induction group (Fig. 3A).
Fig. 3. FOF decreases the function of mature Th1/2 cells in experimental AD models. (A) Mice were stimulated with DNCB for up to 31 days at 2-day intervals, painted with hydrocort cream and FOF (2 and 10%) every other day, as described in Figure legend 1. After sacrifice, the spleen was isolated and photographed to record the morphologic alteration. (B) Splenocytes were isolated from mice in each experimental group and cells (1.0 × 106 cells/ml) were stimulated with anti-CD3 (1 μg/ml) and anti-CD28 (0.5 μg/ml) for 3 days. Mouse IFN-γ and (C) IL-4 were measured in the culture supernatant by ELISA. (D) Cells were permeabilized with T-bet fixation/permeabilization buffer and stained with anti- T-bet-Alexa Fluor® 488 and (E) anti-GATA3-PE and analyzed by FACS. Data are representative of 8 mice per group. The measurements were made in triplicate and are shown as mean ± S.D. (n = 8 mice per group). *P< 0.05; **P< 0.005; and *** P<0.001 compared to mice sensitized with DNCB alone (induction group).
We measured whether FOF suppresses mature Th1 and Th2 cell functions in the experimental AD models. Spleen cells from each group were stimulated with anti-CD3 (1 μg/ ml) and anti-CD28 (0.5 μg/ml) for 3 days. The relative levels of IFN-γ and IL-4 cytokines were measured by ELISA and the expression of the Th1 T-box-expressed-in-T-cell (Tbet) and Th2 GATA-binding protein 3 (GATA3) transcription factors was measured by FACS. Splenocytes from mice treated with 10% FOF produced significantly less IL-4 and IFN-γ compared with the induction group following stimulation with anti-CD3/CD28 (Fig. 3B and C). In addition, 10% FOF treatment decreased T-bet expression from 44.6% of splenocytes (induction group) to 27.4% (10% FOF) or 30.7% (hydrocort cream) and decreased GATA3 expression from 28.8% of splenocytes (induction group) to 17.6% (10% FOF) or 16.4% (hydrocort cream). The 10% FOFtreated group exhibited stronger anti-inflammatory effects on effector T cell inflammatory factors than the 2% FOFtreated group (Fig. 3D and E).
DISCUSSION
In this study, we fermented olive flounder oil and tested its ability to suppress the various allergic inflammatory responses. FOF treatment potently modulates the immune system and the allergic response.
AD mainly appears together with various diseases including eczema, asthma and allergic conjunctivitis (Leung et al., 2004). The symptoms of AD include peripheral eosinophilia, epidermal hyperplasia and tissue remodeling (Li et al., 2010; Li et al., 2005; Nystad et al., 2005; Wahlgren 1999). In the present study, we used the DNCB-induced AD mouse model to investigate the anti-inflammatory effect of FOF. IgE is an crucial target in the treatment of allergy, and signaling through FcεRI can induce histamine release from mast cells, which leads to potent induction of pruritus or edema (Schwartz, 2004). Therefore, we measured whether FOF can reduce serum IgE, histamine hyperproduction and cutaneouse edema. The FOF treatment decreased IgE, histamine levels and edema compared with the induction group. We predicted that the decreased IgE and histamine levels after FOF treatment would lead to alleviation of cutaneous edema. H&E staining and IHC of the ear tissue confirmed that FOF treatment alleviated inflammatory cell infiltration and the expression of TSLP compared with the induction group. Furthermore, the FOF-treated group was not appreciably different from the hydrocort cream treatment group on TSLP hyperproduction.
The spleen plays an crucial role in regulating the immune system and contains a range of immune cells. Also, enlared spleen means a splenomegaly by abnormality of immune system function. We observed the morphologic features of the spleen in AD model. The induction group had markedly enlarged spleens; spleen size was reduced in the FOFtreated groups. Mice in the 10% FOF group had smaller spleens than mice in the 2% FOF group. So, FOF reduced enlarged spleen by alleviating abnormality of immune system function. Th2 cells produce a variety of cytokines, such as IL-4, -5 and -13, and preferentially express GATA3, which is important for Th2 differentiation. Th1 cells produce IFN-γ and T-bet is critical for the differentiation of Th1 cells (Szabo et al., 2000; Zheng and Flavell, 1997). We measured whether FOF treatment regulate the expression of Th1/Th2 associated factors in experimental AD model. FOF decreased IFN-γ and IL-4 levels and the expression of T-bet and GATA3. In summary, FOF had strong inhibitory effects on various experimental AD symptoms. It is not yet clear why FOF has strong immunomodulatory effects. So we are currently trying to identify the inter-relationships between FOF, EPA, DHA and Tregs. Our results suggest that the fermented olive flounder oil may be an effective treatment for the allergic symptoms of AD.
Acknowledgments
This research was supported by a grant from the Korean Ministry of Knowledge and Economy (R0000112).
References
- 1.Al-Shami A., Spolski R., Kelly J., Keane-Myers A., Leonard W.J. A role for TSLP in the development of inflammation in an asthma model. J. Exp. Med. (2005);202:829–839. doi: 10.1084/jem.20050199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bogiatzi S.I., Fernandez I., Bichet J.C., Marloie-Provost M.A., Volpe E., Sastre X., Soumelis V. Cutting edge: Proinflammatory and Th2 cytokines synergize to induce thymic stromal lymphopoietin production by human skin keratinocytes. J. Immunol. (2007);178:3373–3377. doi: 10.4049/jimmunol.178.6.3373. [DOI] [PubMed] [Google Scholar]
- 3.de Vries I.J., Langeveld-Wildschut E.G., van Reijsen F.C., Bihari I.C., Bruijnzeel-Koomen C.A., Thepen T. Nonspecific T-cell homing during inflammation in atopic dermatitis: Expression of cutaneous lymphocyte-associated antigen and Integrin alphaE beta7 on skin-infiltrating T cells. J. Allergy Clin. Immunol. (1997);100:694–701. doi: 10.1016/S0091-6749(97)70175-1. [DOI] [PubMed] [Google Scholar]
- 4.Debes G.F., Bonhagen K., Wolff T., Kretschmer U., Krautwald S., Kamradt T., Hamann A. CC chemokine receptor 7 expression by effector/memory CD4+ T cells depends on antigen specificity and tissue localization during influenza a virus infection. J. Virol. (2004);78:7528–7535. doi: 10.1128/JVI.78.14.7528-7535.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ebner S., Nguyen V.A., Forstner M., Wang Y.H., Wolfram D., Liu Y.J., Romani N. Thymic stromal lymphopoietin converts human epidermal langerhans cells into antigenpresenting cells that induce proallergic T cells. J. Allergy Clin. Immunol. (2007);119:982–990. doi: 10.1016/j.jaci.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 6.Elson C.O., Cong Y., Brandwein S., Weaver C.T., McCabe R.P., Mähler M., Sundberg J.P., Leiter E.H. Experimental models to study molecular mechanisms underlying intestinal inflammation. Ann. N. Y. Acad. Sci. (1998);859:85–95. doi: 10.1111/j.1749-6632.1998.tb11113.x. [DOI] [PubMed] [Google Scholar]
- 7.Ginsberg G.L., Toal B.F. Quantitative approach for incorporating methylmercury risks and omega-3 fatty acid benefits in developing species-specific fish consumption advice. Environ Health Perspect. (2009);117:267–275. doi: 10.1289/ehp.11368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Glimcher L.H., Murphy K.M. Lineage commitment in the immune system: The T helper lymphocyte grows up. Genes Dev. (2000);14:1693–1711. [PubMed] [Google Scholar]
- 9.Isobe N., Suzuki M., Oda M., Tanabe S. Enzymemodified cheese exerts inhibitory effects on allergen permeation in rats suffering from indomethacin-induced intestinal inflammation. Biosci. Biotechnol. Biochem. (2008);72:1740–1745. doi: 10.1271/bbb.80042. [DOI] [PubMed] [Google Scholar]
- 10.Kawakami T., Ando T., Kimura M., Wilson B.S., Kawakami Y. Mast cells in atopic dermatitis. Curr. Opin. Immunol. (2009);21:666–678. doi: 10.1016/j.coi.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kitamura Y., Ito A. Mast cell-committed progenitors. Proc. Natl. Acad. Sci. U.S.A. (2005);102:11129–11130. doi: 10.1073/pnas.0505073102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krauss-Etschmann S., Hartl D., Rzehak P., Heinrich J., Shadid R., Del Carmen Ramirez-Tortosa M., Campoy C., Pardillo S., Schendel D.J., Decsi T., Demmelmair H., Koletzko B.V. Decreased cord blood IL-4, IL-13, and CCR4 and increased TGF-beta levels after fish oil supplementation of pregnant women. J. Allergy Clin. Immunol. (2008);121:464–470. doi: 10.1016/j.jaci.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 13.Kremmyda L.S., Vlachava M., Noakes P.S., Diaper N.D., Miles E.A., Calder P.C. Atopy risk in infants and children in relation to early exposure to fish, oily fish, or long-Chain Omega-3 fatty acids: A systematic review. Clin. Rev. Allergy Immunol. (2011);41:36–66. doi: 10.1007/s12016-009-8186-2. [DOI] [PubMed] [Google Scholar]
- 14.Kwon D.Y., Daily J.W. 3rd, Kim H.J., Park S. Antidiabetic effects of fermented soybean products on type 2 diabetes. Nutr. Res. (2010);30:1–13. doi: 10.1016/j.nutres.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 15.Leitges M., Gimborn K., Elis W., Kalesnikoff J., Hughes M.R., Krystal G., Huber M. Protein kinase C-delta is a negative regulator of antigen-induced mast cell degranulation. Mol. Cell Biol. (2002);22:3970–3980. doi: 10.1128/MCB.22.12.3970-3980.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leung D.Y., Boguniewicz M., Howell M.D., Nomura I., Hamid Q.A. New insights into atopic dermatitis. J. Clin. Invest. (2004);113:651–657. doi: 10.1172/JCI21060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levin T.A., Ownby D.R., Smith P.H., Peterson E.L., Williams L.K., Ford J., Young P., Johnson C.C. Relationship between extremely low total serum IgE levels and rhinosinusitis. Ann. Allergy Asthma Immunol. (2006);97:650–652. doi: 10.1016/S1081-1206(10)61095-2. [DOI] [PubMed] [Google Scholar]
- 18.Li C., Lasse S., Lee P., Nakasaki M., Chen S.W., Yamasaki K., Gallo R.L., Jamora C. Development of atopic dermatitis- like skin disease from the chronic loss of epidermal caspase-8. Proc. Natl. Acad. Sci. U.S.A. (2010);107:22249–22254. doi: 10.1073/pnas.1009751108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li M., Messaddeq N., Teletin M., Pasquali J.L., Metzger D., Chambon P. Retinoid X receptor ablation in adult mouse keratinocytes generates an atopic dermatitis triggered by thymic stromal lymphopoietin. Proc. Natl. Acad. Sci. U.S.A. (2005);102:14795–14800. doi: 10.1073/pnas.0507385102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nystad W., Roysamb E., Magnus P., Tambs K., Harris J.R. A comparison of genetic and environmental variance structures for asthma, hay fever and eczema with symptoms of the same diseases: A study of norwegian twins. Int. J. Epidemiol. (2005);34:1302–1309. doi: 10.1093/ije/dyi061. [DOI] [PubMed] [Google Scholar]
- 21.Ouyang W., Ranganath S.H., Weindel K., Bhattacharya D., Murphy T.L., Sha W.C., Murphy K.M. Inhibition of Th1 development mediated by GATA-3 through an IL-4- independent mechanism. Immunity. (1998);9:745–755. doi: 10.1016/S1074-7613(00)80671-8. [DOI] [PubMed] [Google Scholar]
- 22.Romagnani S. The role of lymphocytes in allergic disease. J. Allergy Clin. Immunol. (2000);105:399–408. doi: 10.1067/mai.2000.104575. [DOI] [PubMed] [Google Scholar]
- 23.Schwartz L.B. Effector cells of anaphylaxis: Mast cells and basophils. Novartis Found Symp. (2004);257:65–74. doi: 10.1002/0470861193.ch6. [DOI] [PubMed] [Google Scholar]
- 24.Seki H., Sasaki T., Ueda T., Arita M. Resolvins as Regulators of the immune system. ScientificWorldJournal. (2010);4:818–831. doi: 10.1100/tsw.2010.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Soumelis V., Reche P.A., Kanzler H., Yuan W., Edward G., Homey B., Gilliet M., Ho S., Antonenko S., Lauerma A., Smith K., Gorman D., Zurawski S., Abrams J., Menon S., McClanahan T., de Waal-Malefyt Rd R., Bazan F., Kastelein R.A., Liu Y.J. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat. Immunol. (2002);3:673–680. doi: 10.1038/nrm910. [DOI] [PubMed] [Google Scholar]
- 26.Stone K.D., Prussin C., Metcalfe D.D. IgE, mast cells, basophils, and eosinophils. J. Allergy Clin. Immunol. (2010);125:S73–S80. doi: 10.1016/j.jaci.2009.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Szabo S.J., Kim S.T., Costa G.L., Zhang X., Fathman C.G., Glimcher L.H. A novel transcription factor, T-Bet, directs Th1 lineage commitment. Cell. (2000);100:655–669. doi: 10.1016/S0092-8674(00)80702-3. [DOI] [PubMed] [Google Scholar]
- 28.Uchida M., Shimizu K., Kurakazu K. Yogurt containing lactobacillus gasseri OLL 2716 (LG21 Yogurt) accelerated the healing of acetic acid-induced gastric ulcer in rats. Biosci. Biotechnol. Biochem. (2010);74:1891–1894. doi: 10.1271/bbb.100287. [DOI] [PubMed] [Google Scholar]
- 29.Wahlgren C.F. Itch and atopic dermatitis: An overview. J. Dermatol. (1999);26:770–779. doi: 10.1111/j.1346-8138.1999.tb02090.x. [DOI] [PubMed] [Google Scholar]
- 30.Zheng W., Flavell R.A. The transcription factor GATA-3 is necessary and sufficient for Th2 cytokine gene expression in CD4 T cells. Cell. (1997);89:587–596. doi: 10.1016/S0092-8674(00)80240-8. [DOI] [PubMed] [Google Scholar]