Abstract
In recent decades, titanium dioxide (TiO2) nanoparticles have been used in various applications, including paints, coatings, and food. However, data are lacking on the toxicological aspects associated with their use. The aim of this study was to assess the inhalation toxicity of TiO2 nanoparticles in rats by using inhalation exposure. Male Wistar rats were exposed to TiO2 nanoparticles for 2 weeks (6 hr/day, 5 days/week) at a mean mass concentration of 11.39 ± 0.31 mg/m3. We performed time-course necropsies at 1, 7, and 15 days after exposure. Lung inflammation and injury were assessed on the basis of the total and individual cell counts in bronchoalveolar lavage fluid (BALF), and by biochemical assays, including an assay for lactate dehydrogenase (LDH). Furthermore, histopathological examination was performed to investigate the lungs and nasal cavity of rats. There were no statistically significant changes in the number of BALF cells, results of biochemical assays of BALF and serum, and results of cytokine analysis. However, we did observe histopathological changes in the nasal cavity tissue. Lesions were observed at post-exposure days 1 and 7, which resolved at post-exposure day 15. We also calculated the actual amounts of TiO2 nanoparticles inhaled by the rats. The results showed that the degree of toxicity induced by TiO2 nanoparticles correlated with the delivered quantities. In particular, exposure to small particles with a size of approximately 20 nm resulted in toxicity, even if the total particle number was relatively low.
Keywords: Titanium dioxide, Nanoparticle, Nose-only exposure, Nasal toxicity, BAL fluid, Lung
INTRODUCTION
Titanium dioxide (TiO2) nanoparticles have been used as a white pigment in paint, a food-coloring agent and a wastewater remediation agent (Nordman and Berlin, 1986; Lomer et al., 2002; Cho et al., 2004). Additionally, they have been widely used as an ultraviolet blocker in sunscreens (Sadrieh et al., 2010). Recently, researchers have investigated whether TiO2 nanoparticles may been also useful as a photocatalyst and as an important material of solar cells. In addition, studies are being conducted to establish new approaches for medical treatment using photosensitive TiO2 nanoparticles (Gurr et al., 2005). TiO2 nanoparticles may be applicable to various coating or surface treatment processes. Therefore, widespread human exposure may occur during the production processes and applications of TiO2 nanoparticles.
Upon inhalation, TiO2 nanoparticles are deposited in all regions of the respiratory system and distributed into other organs through cardiovascular circulation. Previous studies have reported cases of TiO2-induced fibrosis (Bermudez et al., 2004; Driscoll et al., 1991) and lung inflammation (Nemmar et al., 2008; Hhr et al., 2002; Park et al., 2009; van Ravenzwaay et al., 2009). In 2006, the International Agency for Research on Cancer (IARC) classified pigmentgrade TiO2 as a group 2B carcinogen, which may be harmful to humans (Baan, 2007). Furthermore, the Workplace Hazardous Materials Information System (WHMIS) groups TiO2 as D2A, which indicates chemical carcinogenic risk to humans. Therefore, it is necessary to assess the risk of TiO2 nanoparticles and to collect toxicity information, since they are still in use, but possibly carcinogenic.
In this study, we exposed male Wistar rats to dispersed aerosols of TiO2 nanoparticles using a nanoparticle generator (SIBATA, Japan), which was used to distribute a consistent concentration of nanoparticles (11.39 ± 0.31 mg/m3) to animals for 2 weeks (6 h/day, 5 days/week). Considering the potential health effects of nanoparticles, we suggest that examination of chronic effects from exposure is more important than assessment of fetal injuries associated with acute response. For that reason, we conducted this study for 2 weeks and compared our results with those from previous TiO2 inhalation toxicity studies that were carried out for 5 days (Driscoll et al., 1991; van Ravenzwaay et al., 2009; Ma-Hock et al., 2009) or 13 weeks (Bermudez et al., 2002; Bermudez et al., 2004).
MATERIALS AND METHODS
Test material and TiO2 nanoparticle generation. TiO2 (Titanium dioxide, 811X1240) nanoparticles were obtained from KANTO CHEMICAL Corporation (Japan). To generate TiO2 nanoparticles, we dispersed test material in distilled water at a concentration of 0.4% w/w. We generated the test material suspension using a nanoparticle generator connected to a nose-only exposure system (SIBATA, Japan). The nanoparticle generator consisted of 3 main parts, including a nebulizer for spraying test materials, a heater to blow away the solvent of the aerosol, and a neutralizer for removing the charges of the materials. The treated group of animals were exposed to the generated aerosols for 2 weeks (6 h/day, 5 days/week). We determined the target mass concentration of approximately 10 mg/m3 using the scanning mobility particle sizer (SMPS) 3034 (TSI, USA). The control animal group was exposed to clean air only.
Animals and conditions. This study was conducted using 7 week-old male Crl:WI (Han) Wistar rats that were obtained from Orient Bio (Korea). The animals were acclimated for 10 days before we began the study. The rats were maintained in cages at 23 ± 3℃, with relative humidity of 50 ± 10%, and a 12 hour light-dark cycle. The rats were fed rodent diet (Labdiet; PMI Nutrition International Inc., USA) and filtered water ad libitum. The animals were examined for clinical signs once a day for the duration of exposure. After 2 weeks of exposure, we conducted necropsies at 1, 7, and 15 days. Subsequently, we weighed the organs, performed histopathological examination, and analyzed the bronchoalveolar lavage fluid (BALF) and serum for 6~7 animals at each time point (Table 1). This study was carried out in compliance with the Association for Assessment and Accreditation of Laboratory Animal Care.
Table 1.
Study design
| Study day | 1-10 | 11 | 12-16 | 17 | 18-24 | 25 |
|---|---|---|---|---|---|---|
| Study phase | EP | R | R | R | R | R |
| Examinations and their time points | N (Day 1) | N (Day 7) | N (Day 15) | |||
EP = Nose-only exposure for 6 hours a day, R = Recovery period; N = Necropsy, organ weight, histopathology, and BALF.
Biochemistry in BAL fluid and serum. On 1, 7 and 15 days post-exposure, bronchoalveolar lavage fluid (BALF) was collected. The right lung was lavaged 3 times with 15 ml (22 ml/kg body weight) of 0.9% (w/v) saline to obtain the BALF from animals. The BALF samples were centrifuged (600 g, 10 min, 4℃) and their supernatants were analyzed with a FUJI DRI-CHEM FDC3500 (FUJIFILM, Japan) for activities of lactate dehydrogenase (LDH), γ- glutamyltransferase (GGT), and alkaline phosphatase (ALP). We also determined the total cell count in BALF with a Vi-CELL (Beckman Coulter, USA). Differential cell counts were performed on cytocentrifuged preparations and stained with Wright-Giemsa. The blood from abdominal vein was centrifuged (1600 g, 15 min, 4℃) and the resultant supernatants were stored at −80℃ for further biochemical analyses. To be specific, we determined levels of lactate dehydrogenase (LDH), and blood urea nitrogen (BUN), total protein (TP) in serum using a FUJI DRI-CHEM FDC3500.
Histopathology. Necropsies were performed at 1, 7, and 15 days post-exposure. Rats were euthanized by isoflurane anesthesia. Blood was collected from abdominal vein. We obtained the lungs, livers, and nasal cavities from animals after necropsy and measured the organs weights. The lungs and nasal cavities were fixed in a 10% natural formalin solution for further histopathological diagnosis. Paraffinembedded tissues were sectioned, stained with hematoxylin and eosin (H&E), and examined by light microscopy.
Statistical analysis. Each of the experimental values was compared to the corresponding control value for each time point using Student’s t-test. Comparisons were considered statistically significant when p < 0.05.
RESULTS
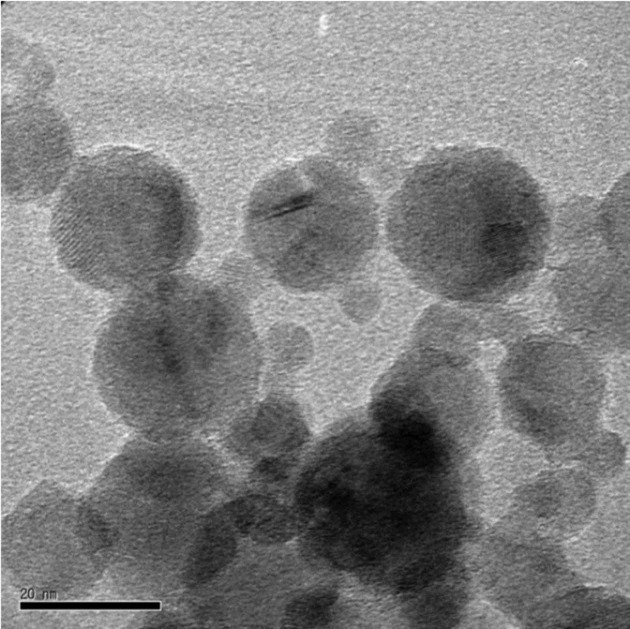
Test material analysis. TiO2 nanoparticles consisted of anatase as the major crystal phase from the XRD (X-ray diffractometry) pattern (Data not shown). Briefly, we obtained the volume ratio of the two crystal phases, 100 of anatase phase and 5.8 of rutile phase. Thus, it was noted that approximately 5.5% volume of the rutile phase existed in the nanoparticles. Furthermore, we calculated the mean crystallite size of TiO2 nanoparticles using the full width at half maximum of peaks and the Scherrer formula. Anatase and rutile phases revealed mean crystallite sizes of 52.6 nm and 60.9 nm, respectively. In addition, TiO2 nanoparticles seemed not to be charged, but instead neutral from fourier transform infrared spectroscopy (FT-IR) spectrum (Data not shown). Fig. 1 shows the morphological characteristics of TiO2 nanoparticles, which exhibited a wide range of sizes with a few particles up to 100 nm. We also observed that the particles have mostly spherical shapes and the presence of some agglomerated nanoparticles.
Fig. 1. TEM micrograph of TiO2 nanoparticles obtained at the magnification of 200,000 ×. The morphological characteristics of TiO2 nanoparticles are shown, which exhibit a wide range of size, with a few particles up to 100 nm. The particle shape was mostly spherical. Agglomerated TiO2 nanoparticles were also found. TEM: Transmission electron microscopy.
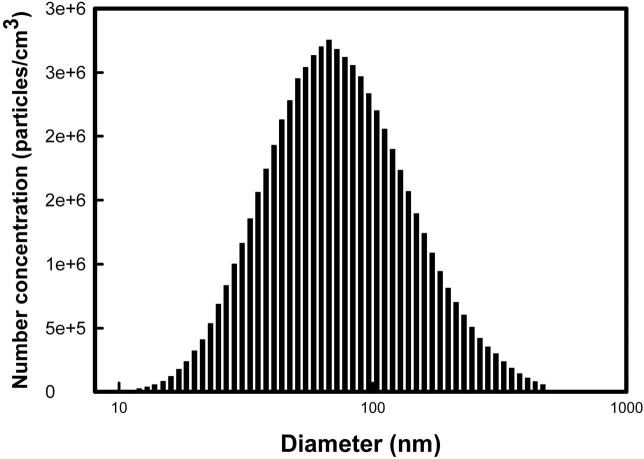
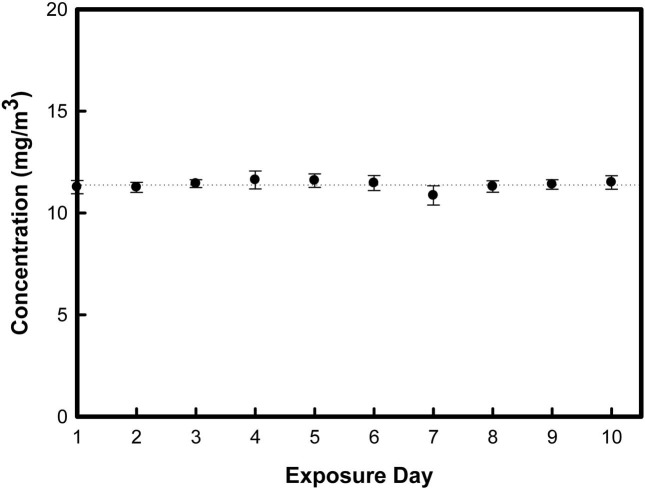
Particle concentration in the chamber. We also evaluated the size distribution of nanoparticles in the chamber using a SMPS during inhalation exposure periods. Fig. 2 shows the representative data from this analysis. By controlling the generation of the airflow and the dilution of the airflow of the nanoparticle generator, we could maintain the mean mass concentration of TiO2 nanoparticles as 11.39 ± 0.31 mg/m3 (Fig. 3). Furthermore, we obtained the geometric mean diameter (GMD) and total number concentration during inhalation exposure periods. The mean GMD (nm) ± geometrical standard deviation (GSD) and total number concentration was determined to be 79.19 ± 4.41 nm and 1.8 × 106 particles/cm3, respectively (Data not shown).
Fig. 2. The size distribution was monitored using the SMPS during inhalation exposure periods. It shows the representative distribution of the sizes of TiO2 nanoparticles. SMPS: Scanning mobility particle sizer.
Fig. 3. In the nose-only inhalation chamber, mean mass concentration of TiO2 nanoparticles were determined to be 11.39 ± 0.31 mg/m3. Error bar means standard deviation of each exposure day.
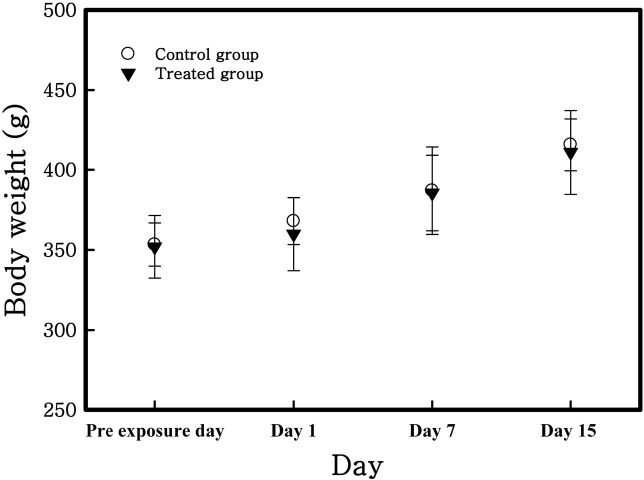
Animal observations, body weights, and organ weights. There was no significant clinical sign induced by TiO2 nanoparticles for the study durations. Body weight was measured prior to inhalation exposure and at 1, 7 and 15 days post-exposure. In all groups, body weights were increased with the passage of time (Fig. 4) but there were no statistically significant changes. Table 2 shows that calculated relative organ weights (i.e. lung, liver and spleen) to mean body weight of each group. Increased weight of the spleen of all groups was caused by increasing their body weight. There were no significant changes of liver and lung weights.
Fig. 4. Body weight change of rats exposed to TiO2 nanoparticles. There were no significant differences between the control and treated group. The data are presented as the mean (g) ± SD.
Table 2.
Absolute and relative organ weights
| Day 1 post-exposure | Day 7 post-exposure | Day 15 post-exposure | ||||
|---|---|---|---|---|---|---|
| Control | Treated | Control | Treated | Control | Treated | |
| Necropsy body weight | 367.91 ± 14.61 | 359.87 ± 22.81 | 386.97 ± 27.29 | 385.48 ± 23.53 | 415.66 ± 16.32 | 410.95 ± 26.26 |
| Lung | ||||||
| Absolute | 0.46 ± 0.05 | 0.47 ± 0.04 | 0.47 ± 0.07 | 0.47 ± 0.04 | 0.49 ± 0.05 | 0.49 ± 0.02 |
| Relative | 0.12 ± 0.01 | 0.13 ± 0.01 | 0.12 ± 0.01 | 0.12 ± 0.02 | 0.12 ± 0.01 | 0.12 ± 0.01 |
| Liver | ||||||
| Absolute | 13.52 ± 1.60 | 13.11 ± 1.56 | 13.25 ± 2.79 | 14.73 ± 1.20 | 14.28 ± 0.89 | 14.59 ± 1.26 |
| Relative | 3.67±0.43 | 3.64 ± 0.43 | 3.42 ± 0.72 | 3.82 ± 0.31 | 3.44 ± 0.21 | 3.55 ± 0.31 |
| Spleen | ||||||
| Absolute | 0.79 ± 0.08 | 0.75 ± 0.11 | 0.85 ± 0.17 | 0.94 ± 0.16 | 1.03 ± 0.05 | 0.93 ± 0.10 |
| Relative | 0.22 ± 0.02 | 0.21 ± 0.03 | 0.22 ± 0.04 | 0.24 ± 0.04 | 0.25 ± 0.01 | 0.23 ± 0.02 |
Note: All values are presented as the mean (g) ± standard deviation for the indicated animal groups.
BAL fluid cytology and biochemical parameters in BAL fluid and serum. Results of BAL fluid cytology show that the total cell number and macrophage value were highest at 1 day post-exposure (Table 3). In treated group, total cell number and macrophage value were higher than control group of each time point. However there was no statistical significance between control and treated group. In addition, there were no differences in biochemical parameters of LDH, ALP, and GGT in BAL fluid and LDH, BUN, and TP in serum of all rats (Table 4).
Table 3.
BALF cytology of rats exposed to TiO2 nanoparticles
| Cell type | Day 1 post-exposure | Day 7 post-exposure | Day 15 post-exposure | |||
|---|---|---|---|---|---|---|
| Control | Treated | Control | Treated | Control | Treated | |
| Total cell | 5.246 | 5.361 | 4.166 | 4.230 | 4.650 | 4.780 |
| Macrophage | 5.230 | 5.356 | 4.153 | 4.221 | 4.640 | 4.716 |
| Neutrophil | 0.003 | 0.004 | 0.008 | 0.005 | 0.004 | 0.008 |
| Lymphocyte | 0.013 | 0.002 | 0.005 | 0.004 | 0.006 | 0.057 |
Note: Rats were treated with 0 (control) or 11.39 mg/m3 (Treatment) TiO2 nanoparticles by inhalation for 2 weeks. Lung lavage was performed on 1,7, and 15 days post-exposure.
Mean values {(Cell counts/ml BALF) × 105} of the indicated animal groups are shown.
Table 4.
Biochemical parameters in BALF and serum after exposure of TiO2 nanoparticles
| Day 1 post-exposure | Day 7 post-exposure | Day 15 post-exposure | |||||
|---|---|---|---|---|---|---|---|
| Control | Treated | Control | Treated | Control | Treated | ||
| BALF | LDH | 1.33 ± 0.82 | 1.14 ± 0.38 | 2.00 ± 1.41 | 1.67 ± 1.63 | 1.75 ± 5.96 | 1.50 ± 2.07 |
| GGT | 10.00 ± 2.58 | 8.86 ± 1.07 | 9.86 ± 2.41 | 9.00 ± 0.89 | 8.67 ± 1.03 | 8.00 ± 1.67 | |
| ALP | 17.57 ± 3.55 | 14.57 ± 3.60 | 16.71 ± 4.23 | 19.83 ± 3.25 | 19.67 ± 6.95 | 25.67 ± 6.62 | |
| Day 1 post-exposure | Day 7 post-exposure | Day 15 post-exposure | |||||
| Control | Treated | Control | Treated | Control | Treated | ||
| Serum | LDH | 820.86 ± 62.34 | 771.33 ± 154.30 | 703.57 ± 171.52 | 728.00 ± 181.43 | 641.50 ± 232.23 | 729.50 ± 87.55 |
| BUN | 13.73 ± 0.69 | 15.29 ± 2.29 | 17.29 ± 6.10 | 14.22 ± 1.14 | 19.35 ± 4.39 | 20.48 ± 4.48 | |
| TP | 5.17 ± 0.19 | 5.41 ± 0.19 | 5.17 ± 0.18 | 5.13 ± 0.19 | 5.40 ± 0.18 | 5.52 ± 0.16 | |
Note: All values are presented as the mean (U/L) ± standard deviation for the indicated animal groups.
BALF: Bronchoalveolar lavage fluid, LDH: Lactate dehydrogenase, ALP: Alkaline phosphatase, GGT: γ-glutamyltransferase, BUN: Blood urea nitrogen, TP: Total protein
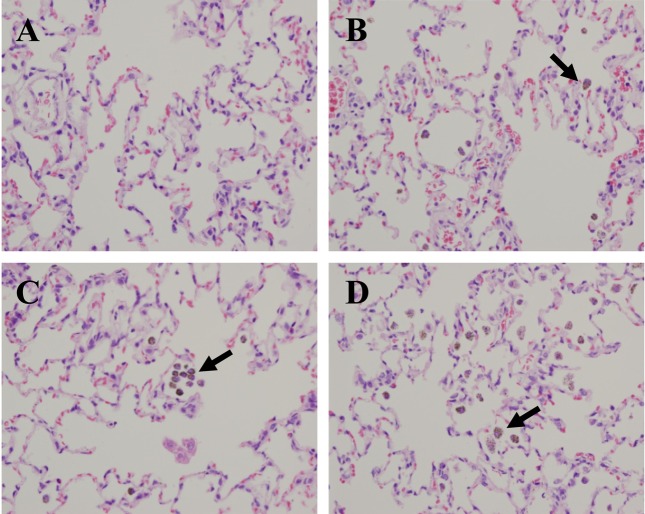
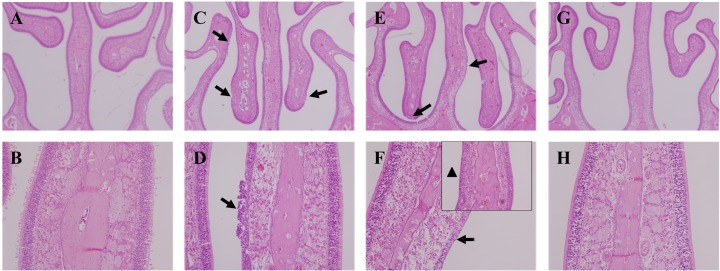
Histopathological examination. Upon examination of the lung tissues of all treated rats, we observed numerous brown pigmented macrophages in alveoli until 15 days post-exposure (Fig. 5). At 1 day post-exposure, we found olfactory epithelium degeneration/regeneration with inflammatory cell infiltration on the nasal septum and ethmoid turbinate of the treated rats. Besides, we observed basal cell proliferation on the ethmoid turbinate at 7 day post-exposure. However, these lesions were not observed at 15 days post-exposure (Fig. 6).
Fig. 5. Histopathological examination was performed in the lungs of rats exposed to TiO2 nanoparticles for 2 weeks. (A) Lung of control rat. (B) Day 1 post-exposure. (C) Day 7 postexposure. (D) Day 15 post-exposure. Arrows indicate pigmented macrophages in alveoli. Hematoxylin and eosin, 400 × magnification.
Fig. 6. Histopathological examination of the nasal cavities of rats exposed to TiO2 nanoparticles for 2 weeks. (A, B) Nasal cavity of control rat. (C, D) Nasal cavity of rat sacrificed at day 1 post-exposure. Moderate olfactory degeneration/regeneration with inflammatory cell infiltration was observed on the nasal septum and ethmoid turbinate (arrows) (E, F) Nasal cavity of rat sacrificed at day 7 postexposure. Besides, olfactory degeneration/regeneration was observed on the nasal septum and ethmoid turbinate (arrows). Also, basal cell proliferation was observed on the ethmoid turbinate (arrow head). (G, H) Nasal cavity of rat sacrificed at day 15 post-exposure. Lesions were not observed. Hematoxylin and eosin, A, C, E, G: 40 × magnification; B, D, F, H: 200 × magnification.
DISCUSSION
The aim of this study is to examine the influence of TiO2 nanoparticles on rats that were exposed using a system for nose-only exposure. Rats in the treated group were exposed to a mass concentration of 11.39 mg/m3 for 2 weeks; control rats were exposed to clean air only. We did not observed changes in clinical signs, body weights and organ weight, cells of the BALF, biochemical parameters and cytokines on BALF and serum during the study. In general, total cell counts, LDH, TP levels and other enzyme activities represent parameters of cell injury in toxicity studies including nanoparticles (Driscoll et al., 1991; Bermudez et al., 2002; Bermudez et al., 2004; van Ravenzwaay et al., 2009; Ma-Hock et al., 2009). In this study, we did not observe any significant differences between the control and treated groups in terms of these factors. Also, IL-6, an important immune modulation mediator, is generally known to function in acute inflammation. As cell activators, IL-4 and IL-10 are cytokines of the Th2-type. Specially, IL-10 is related to the fibrotic process and chronic inflammation (Park et al., 2009). In this study, we did not observe significant differences in levels of IL-4, IL-6, or IL-10 between the control and treated groups on 1, 7, and 15 days postexposure (Data not shown). However, upon examination of stained BALF cell (1000 × magnification) with a microscope, we found phagocytosed macrophages of all the treated rats (Data not shown). This result suggests that the concentration of TiO2 nanoparticles that we exposed to rat was not high enough to activate the immune cells. Instead, the concentration we used was sufficient to control macrophages in alveoli. Bermudez et al. (2002) also noted no signs of inflammation upon histopathological observation of 6-week-old female rats exposed to 10 mg/m3 TiO2 concentration for 13 weeks (6 hr/day, 5 days/week). However, other studies indicated toxicological changes when nanoparticles were phagocyted by macrophage. It is the case that exposure period is sufficiently long and particle concentration is high to cause inflammation.
The actual delivered amounts of TiO2 nanoparticles that are exposed to rats can be calculated by multiplying the aerosol concentration of TiO2 nanoparticles by the amount of inhaled air by rats, which was previously reported by Alexander et al. (2008). Table 5 shows the estimation data that we calculated by this formula using the aerosol concentration of TiO2 nanoparticles and the animals’ mean body weights from the data of several inhalation studies. From this table, we concluded that the exposure amount used in this study is comparatively low (10.27 mg/rat) in comparison to other studies that indicated toxicity. In this method, we concluded an aspect of correspondence between the actual exposure levels and toxicity degree. When the rats inhaled higher amounts of nanoparticle, the toxic responses were more severe. In addition, we have to consider the size of nanoparticle. We measured that mean particle size of approximately 80 nm in actual exposure atmosphere during study period. As we already know, the toxicity increases as the size of nanoparticles decreases (Sager et al., 2008) due to the high reactivity between nanoparticles with large surface area and because materials of the body (Oberdöster et al., 2005; Warheit et al., 2004; Donaldson et al., 2006; Warheit et al., 2006). In the study carried out by Ma-Hock et al. (2009), these results represent more toxic than other studies because of their small size.
Table 5.
The delivered amounts of TiO2 nanoparticles to rats
| Reference | Particle size (nm) | Aerosol concentration (mg/m3) | Exposure time (min) | Delivered TiO2 amounts (mg/rat)a) | Degree of toxicity |
|---|---|---|---|---|---|
| Bermudez (2004) | 21 | 0.52 | 23400 | 0.63 | No histopathological lesions in lung, No changes in BALF cell parameter or lung burdens |
| Lan Ma-Hock (2009) | 25.1 | 2.4 | 1800 | 1.08 | No effect on the BALF parameters |
| Bermudez (2004) | 21 | 2.1 | 23400 | 2.56 | No histopathological lesions in lungs, No changes in BALF cell parameter or lung burdens |
| Lan Ma-Hock (2009) | 25.1 | 12.1 | 1800 | 5.45 | Increase in BALF cell counts, total protein levels, and enzyme activities |
| This study | 79 | 11.39 | 3600 | 10.27 | No effect on the BALF parameters and biochemistry in BALF and serum, Mild degeneration/ regeneration of olfactory in nasal cavity, lung infiltration of macrophage |
| Bermudez (2002) | Pigment grade | 9.6 | 23400 | 11.72 | No response to inflammation |
| Bermudez (2004) | 21 | 10.5 | 23400 | 12.82 | Increased number of macrophages and neutrophils in BALF, Pulmonary particle overload, epithelial and fibroproliferative changes |
| Kevin E. Driscoll (1991) | Pigment grade | 51.1 | 1800 | 13.59 | No changes in BALF biochemistry, cellular parameter, or histopathological examination. |
| Lan Ma-Hock (2009) | 25.1 | 50.0 | 1800 | 22.53 | Increase in lung weight, BALF cell counts, total protein levels, and enzyme activities Histopathological lesions in the nasal epithelium |
| Ranvenzwaay (2009) | 20~30 | 88.0 | 1800 | 39.66 | Increase in lung weight, neutrophilic inflammation, and activation of macrophages in the lung |
| Bermudez (2002) | Pigment grade | 47.7 | 23400 | 58.22 | Increase in BALF cell number, LDH level in BALF |
| Bermudez (2002) | Pigment grade | 239.1 | 23400 | 291.85 | Increase in BALF cell number, LDH and TP levels in BALF, and epithelial and fibroproliferative changes |
a) Actual delivered TiO2 amounts (mg/kg) = (Aerosol concentration × RMV× Exposure time). RMV: respiratory minute volume (L/min) = 0.608 × BW(kg)0.852.
Note: Delivered TiO2 amounts were calculated according to the article by Alexander et al. (2008). Reference of the body weight was according to Bermudez et al.: www.Lapanimal.co.kr.
We could not observe any changes in biochemical parameters. However, we observed that macrophages infiltrated TiO2 nanoparticles in alveoli of treated rats until 15 days post-exposure. These findings are in line with the results of a previous inhalation study by Bermudez et al. (2004), which showed degeneration and thickness of lung epithelial cells and particle-laden macrophages of the alveolar region. In our study, histopathological examination of nasal cavity of rats at 1 day post-exposure revealed that TiO2 nanoparticles induced olfactory epithelium degeneration/regeneration which was recovered by 15 days post-exposure. Several studies have observed toxicity in the nasal cavity of animals after exposure to nanoparticles. For instance, Tin-Tin-Win-Shwe et al. (2006) observed deposited carbon black nanoparticles in the nasal cavities of BALB/c mice after intranasal instillation once a week for 4 weeks. It has been also reported that ultrafine silver nanoparticles were deposited on the posterior portion of nasal cavities of female Fischer rats after exposure (Takenaka et al., 2001). Furthermore, the previous report of Korea National Toxicology Program, KNTP (2005) demonstrated that ICR mice subjected to nose-only exposure with amorphous silica for 4 weeks exhibited focal histopathological changes in their nasal cavities. In the report, biochemical changes (LDH, total protein, BUN, glucose, total bilirubin and etc.) were observed. In another study including head-nose exposure with nano- TiO2 at 88 mg/m3, particles were observed in the olfactory epithelium of nasal cavities (van Ravenzwaay et al., 2009). Additionally, there were studies that showed histopathological lesions in the nasal epithelium of rats after exposure to TiO2 nanoparticles at concentration of 50 mg/m3 (Ma-Hock et al., 2009) and increases in the incidence of pneumonia, trachitis and rhinitis with squamous metaplasia in the anterior nasal cavity of CD rats exposed TiO2 for 2 years (Lee et al., 1985).
Compared to delivered amount of TiO2 nanoparticle to rats in the table, it is apparent that the table represents doseresponse toxicological changes. In our study, where TiO2 was exposed to ~11 mg/m3 for 2 weeks, we observed histopathological lesions of nasal cavity of treated rats. Among the studies showing toxicity of nasal cavity, it is related to the exposure concentration of inhaled nanoparticles whether the results represent biochemical changes or not.
Inhaled nanoparticles into the body contact with the nasal cavity first and this area is open to outer space. Deposited nanoparticles in the nasal cavity have potential toxicity; they could be translocated to other organs, especially the brain. A previous study reported that TiO2 nanoparticles translocated into the central nervous system and potentially caused lesions of the brain (Wang et al., 2008). This study is in agreement with other previous studies that showed, following inhalation exposure, nanoparticles traveled via the nasal nerves to the brain (Kreuter et al., 2002; Oberdörster et al., 2004). Other studies have also indicated that the olfactory route may be an important transfer route for inhaled nanoparticles to the central nervous system (Oberdrster et al., 2005; KNTP, 2005; Hunter and Undem, 1999; Utell et al., 2002). Therefore, we need to consider the nasal cavity for accomplishment the study of inhalation toxicity.
We observed nasal and pulmonary toxicity which was reversible at the concentration approximately 11 mg/m3 of TiO2 nanoparticles and without any other biochemical changes. This study can serve as an example of investigation of the influence of TiO2 nanoparticles of rats upon nose-only exposure, and can be used to guide future studies designed to estimate nanotoxicity and examine other similar nanoparticles.
Acknowledgments
This study was supported by the Ministry of Knowledge Economy for General Project grant of the establishment of the International Inhalation Toxicology Evaluation technology of Korea Institute of Toxicology.
References
- 1.Alexander D.J., Collins C.J., Coombs D.W., Gilkison I.S., Hardy C.J., Healey G., Karantabias G., Johnson N., Karlsson A., Kilgour J.D., McDonald P. Association of inhalation toxicologists (AIT) working party recommendation for standard delivered dose calculation and expression in non-clinical aerosol inhalation toxicology studies with pharmaceuticals. Inhal. Toxicol. (2008);20:1179–1189. doi: 10.1080/08958370802207318. [DOI] [PubMed] [Google Scholar]
- 2.Baan R.A. Carcinogenic hazards from inhaled carbon black, titanium dioxide, and talc not containing asbestos or asbestiform fibers: Recent evaluations by an iarc monographs working group. Inhal. Toxicol. (2007);19(Suppl 1):213–228. doi: 10.1080/08958370701497903. [DOI] [PubMed] [Google Scholar]
- 3.Bermudez E., Mangum J.B., Asgharian B., Wong B.A., Reverdy E.E., Janszen D.B., Hext P.M., Warheit D.B., Everitt J.I. Long-term pulmonary responses of three laboratory rodent species to subchronic inhalation of pigmentary titanium dioxide particles. Toxicol. Sci. (2002);70:86–97. doi: 10.1093/toxsci/70.1.86. [DOI] [PubMed] [Google Scholar]
- 4.Bermudez E., Mangum J.B., Wong B.A., Asgharian B., Hext P.M., Warheit D.B., Everitt J.I. Pulmonary responses of mice, rats, and hamsters to subchronic inhalation of ultrafine titanium dioxide particles. Toxicol. Sci. (2004);77:347–357. doi: 10.1093/toxsci/kfh019. [DOI] [PubMed] [Google Scholar]
- 5.Cho M., Chung H., Choi W., Yoon J. Linear correlation between inactivation of E. coli and OH radical concentration in TiO2 photocatalytic disinfection. Water Res. (2004);38:1069–1077. doi: 10.1016/j.watres.2003.10.029. [DOI] [PubMed] [Google Scholar]
- 6.Donaldson K., Aitken R., Tran L., Stone V., Duffin R., Forrest G., Alexander A. Carbon nanotubes: A review of their properties in relation to pulmonary toxicology and workplace safety. Toxicol. Sci. (2006);92:5–22. doi: 10.1093/toxsci/kfj130. [DOI] [PubMed] [Google Scholar]
- 7.Driscoll K.E., Lindenschmidt R.C., Maurer J.K., Perkins L., Perkins M., Higgins J. Pulmonary response to inhaled silica or titanium dioxide. Toxicol. Appl. Pharmacol. (1991);111:201–210. doi: 10.1016/0041-008X(91)90024-9. [DOI] [PubMed] [Google Scholar]
- 8.Gurr J.R., Wang A.S., Chen C.H., Jan K.Y. Ultrafine titanium dioxide particles in the absence of photoactivation can induce oxidative damage to human bronchial epithelial cells. Toxicology. (2005);213:66–73. doi: 10.1016/j.tox.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 9.Höhr D., Steinfartz Y., Schinsm R.P., Knaapen A.M., Martra G., Fubini B., Borm P.J. The surface area rather than the surface coating determines the acute inflammatory response after instillation of fine and ultrafine TiO2 in the rat. Int. J. Hyg. Environ. Health. (2002);205:239–244. doi: 10.1078/1438-4639-00123. [DOI] [PubMed] [Google Scholar]
- 10.Hunter D.D., Undem B.J. Identification and substance P content of vagal afferent neurons innervating the epithelium of the guinea pig trachea. Am. J. Respir. Crit. Care Med. (1999);159:1943–1948. doi: 10.1164/ajrccm.159.6.9808078. [DOI] [PubMed] [Google Scholar]
- 11.Kreuter J., Shamenkov D., Petrov V., Ramge P., Cychutek K., Koch-Brandt C., Alyautdin R. Apolipoproteinmediated transport of nanoparticle-bound drugs across the blood-brain barrier. J. Drug Target. (2002);10:317–325. doi: 10.1080/10611860290031877. [DOI] [PubMed] [Google Scholar]
- 12.Kwon J.T., Kim H., Hwang S.T., Yun H.J., Kim D.S., Choi M.S., Han D.Y., Kang Y.W., Yun T.J., Lee J.K., Cho M.H. Inhalation Toxicology of nanoparticle. The Annual Report of KNTP; (2005). pp. 487–507. [Google Scholar]
- 13.Lee K.P., Trochinowicz H.J., Reinhardt C.F. Pulmonary response of rats exposed to titanium dioxide (TiO2) by inhalation for two years. Toxicol. Appl. Pharmacol. (1985);79:179–192. doi: 10.1016/0041-008X(85)90339-4. [DOI] [PubMed] [Google Scholar]
- 14.Lomer M.C., Thompson R.P., Powell J.J. Fine and ultrafine particles of the diet: influence on the mucosal immune response and association with Crohn’s disease. Proc. Nutr. Soc. (2002);61:123–130. doi: 10.1079/PNS2001134. [DOI] [PubMed] [Google Scholar]
- 15.Ma-Hock L., Burkhardt S., Strauss V., Gamer A.O., Wiench K., van Ravenzwaay B., Landsiedel R. Development of a short-term inhalation test in the rat using nano-titanium dioxide as a model substance. Inhal.Toxicol. (2009);21:102–118. doi: 10.1080/08958370802361057. [DOI] [PubMed] [Google Scholar]
- 16.Nemmar A., Melghit K., Ali B.H. The acute proinflammatory and prothrombotic effects of pulmonary exposure to rutile TiO2 nanorods in rats. Exp. Biol. Med. (Maywood) (2008);233:610–619. doi: 10.3181/0706-RM-165. [DOI] [PubMed] [Google Scholar]
- 17.Nordman H., Berlin M., Friberg L., Nordberg G.F., Vouk V.B. Titanium: in Handbook on the Toxicology of Metals, vol. II. Elsevier; Amsterdam: (1986). pp. 595–609. [Google Scholar]
- 18.Oberdörster G., Oberdörster E., Oberdörster J. Nanotoxicology: An emerging discipline evolving from studies of ultrafine particles. Environ. Health Perspect. (2005);113:823–839. doi: 10.1289/ehp.7339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oberdörster G., Sharp Z., Atudorei V., Elder A., Gelein R., Kreyling W., Cox C. Translocation of inhaled ultrafine particles to the brain. Inhal. Toxicol. (2004);16:437–445. doi: 10.1080/08958370490439597. [DOI] [PubMed] [Google Scholar]
- 20.Park E.J., Yoon J., Choi K., Yi J., Park K. Induction of chronic inflammation in mice treated with titanium dioxide nanoparticles by intratracheal instillation. Toxicology. (2009);260:37–46. doi: 10.1016/j.tox.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 21.Sadrieh N., Wokovich A.M., Gopee N.V., Zheng J., Haines D., Parmiter D., Siitonen P.H., Cozart C.R., Patri A.K., McNeil S.E., Howard P.C., Doub W.H., Buhse L.F. Lack of significant dermal penetration of titanium dioxide from sunscreen formulations containing nano- and submicron-size TiO2 particles. Toxicol. Sci. (2010);115:156–166. doi: 10.1093/toxsci/kfq041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sager T.M., Kommineni C., Castranova V. Pulmonary response to intratracheal instillation of ultrafine versus fine titanium dioxide: role of particle surface area. Part. Fibre Toxicol. (2008);5:17. doi: 10.1186/1743-8977-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takenaka S., Karg E., Roth C., Schulz H., Ziesenis A., Heinzmann U., Schramel P., Heyder J. Pulmonary and systemic distribution of inhaled ultrafine silver particles in rats. Environ. Health Perspect. (2001);109(Suppl 4):547–551. doi: 10.1289/ehp.01109s4547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Win-Shwe Tin-Tin, Yamamoto S., Ahmed S., Kakeyama M., Kobayashi T., Fujimaki H. Brain cytokine and chemokine mRNA expression in mice induced by intranasal instillation with ultrafine carbon black. Toxicol. Lett. (2006);163:153–160. doi: 10.1016/j.toxlet.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 25.Utell M.J., Frampton M.W., Zareba W., Devlin R.B., Cascio W.E. Cardiovascular effects associated with air pollution: potential mechanisms and methods of testing. Inhal. Toxicol. (2002);14:1231–1247. doi: 10.1080/08958370290084881. [DOI] [PubMed] [Google Scholar]
- 26.van Ravenzwaay B., Landsiedel R., Fabian E., Burkhardt S., Strauss V., Ma-Hock L. Comparing fate and effects of three particles of different surface properties: Nano-TiO2, pigmentary TiO2 and quartz. Toxicol. Lett. (2009);186:152–159. doi: 10.1016/j.toxlet.2008.11.020. [DOI] [PubMed] [Google Scholar]
- 27.Wang J., Chen C., Liu Y., Jiao F., Li W., Lao F., Li Y., Li B., Ge C., Zhou G., Gao Y., Zhao Y., Chai Z. Potential neurological lesion after nasal instillation of TiO2 nanoparticles in the anatase and rutile crystal phases. Toxicol. Lett. (2008);183:72–80. doi: 10.1016/j.toxlet.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 28.Warheit D.B., Laurence B.R., Reed K.L., Roach D.H., Reynolds G.A., Webb T.R. Comparative pulmonary toxicity assessment of single-wall carbon nanotubes in rats. Toxicol. Sci. (2004);77:117–125. doi: 10.1093/toxsci/kfg228. [DOI] [PubMed] [Google Scholar]
- 29.Warheit D.B., Webb T.R., Sayes C.M., Colvin V.L., Reed K.L. Pulmonary instillation studies with nanoscale TiO2 rods and dots in rats: Toxicity is not dependent upon particle size and surface area. Toxicol. Sci. (2006);91:227–236. doi: 10.1093/toxsci/kfj140. [DOI] [PubMed] [Google Scholar]