Abstract
Quantum dots (QDs) have received considerable attention due to their potential role in photosensitization during photodynamic therapy. Although QDS are attractive nanomaterials due to their novel and unique physicochemical properties, concerns about their toxicity remain. We suggest a combination strategy, CdSe/ZnS QDs together with curcumin, a natural yellow pigment from turmeric, to reduce QD-induced cytotoxicity. The aim of this study was to explore a potentially effective cancer treatment: co-exposure of HL-60 cells and human normal lymphocytes to CdSe/ZnS QDs and curcumin. Cell viability, apoptosis, reactive oxygen species (ROS) generation, and DNA damage induced by QDs and/or curcumin with or without ultraviolet A (UVA) irradiation were evaluated in both HL-60 cells and normal lymphocytes. In HL-60 cells, cell death, apoptosis, ROS generation, and single/double DNA strand breaks induced by QDs were enhanced by treatment with curcumin and UVA irradiation. The protective effects of curcumin on cell viability, apoptosis, and ROS generation were observed in normal lymphocytes, but not leukemia cells. These results demonstrated that treatment with QD combined with curcumin increased cell death in HL-60 cells, which was mediated by ROS generation. However, curcumin acted as an antioxidant in cultured human normal lymphocytes.
Keywords: CdSe/ZnS QDs, Curcumin, Apoptosis, Reactive oxygen species, DNA damage
INTRODUCTION
Nanomedicine, integrated nanotechnology and medicine, is used to improve human health and has become one of the most promising and attractive nanotechnology fields (1,2). Quantum dots (QDs), semiconductor nanocrystals composed of a quantum dot core and a shell, have unique optical and physicochemical properties, such as wide absorption spectra, narrow emission bands, high photostability, and photoluminescence (3,4). Because of their novel characteristics, QDs have become very attractive nanomaterials for bioimaging and cancer therapy in nanomedicine (2). However, cadmium-containing QDs (CdSe QDs) have raised concerns regarding toxicity. Although the toxicity of CdSebased QDs can be somewhat reduced by incorporating a protective zinc-sulfide (ZnS) inorganic shell, the release of Cd2+ ions eventually leads to toxic effects on biological systems (5). Furthermore, it was recently reported that the cytotoxicity of CdTe/CdS (core-shell) and CdTe/CdS/ZnS (core-shell-shell) QDs is also due to intracellular distribution of QDs in cells and the associated nanoscale effects (6). Previously, we showed that CdSe core/ZnS shell QDs induced cyto- and genotoxicity in human normal lymphocytes as well as human lung cancer cells. Specifically, QD phototoxicity was remarkably increased following UV irradiation via reactive oxygen species (ROS) generation, single- and double-stranded DNA damage, and finally, apoptotic or/and necrotic cell death, which implies potential photodynamic therapy (PDT) applications for lung cancer cells (2). Numerous studies have strived to reduce QD toxicity and render them water-soluble for biological applications; potential methods of reducing toxicity include synthesis of QDgelatin nanocomposites, cadmium-free QDs, and biofunctionalized (polymer or peptide conjugates) QDs (5,7,8).
Curcumin, a natural yellow pigment isolated from turmeric (Curcuma longa), is an anti-inflammatory, antioxidant, antimicrobial, and anticancer agent, and has long been used as a food additive and medicinal agent (9-11). Curcumin exerts dual actions, both as an antioxidant and cytotoxicant (12). In addition to its cytotoxic anticancer effects, curcumin reduces oxygen free radicals, prevents lipid peroxidation, and attenuates DNA damage (10). Curcumin has been investigated extensively in human melanoma, and head and neck, breast, colon, pancreatic, prostate and ovarian cancers. Curcumin exerts its anticancer effects by stimulating apoptosis, regulating cellular growth, suppressing nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) activation, modulating autophagy, and inhibiting tumor angiogenesis (13). Among these mechanisms, curcumin-induced apoptosis is the main pathway targeted in anticancer strategies. Apoptosis is targeted in various ways, including decreasing the expression of antiapoptotic members of the Bcl-2 family and increasing the expression of proapoptotic members (e.g., Bax, caspase-3, -8, and -9) (14). Curcumin, which possesses photosensitizing and phytochemical properties and has chemopreventive potential, has gained attention for use in developing adjuvant chemotherapies or PDT to increase therapeutic efficacy and reduce side effects (13-15). However, the cytotoxic and preventive effect of curcumin against nanoparticle-induced toxicity on cancer cells and normal cells has not been reported.
Effective cancer treatment protocols that combine photoactive nanoparticles and natural products without damaging normal cells are of great interest. In this study, the effect of a combination of CdSe/ZnS QDs and curcumin under UVA irradiation on apoptotic cell death in HL-60 cells and the protective effect of curcumin against CdSe/ZnS QDinduced cyto/genotoxicity in human normal lymphocytes were examined.
MATERIALS AND METHODS
Cell culture. A human promyelocytic leukemia cell line (HL-60) was obtained from the Korean Cell Line Bank (Seoul, Korea) and cultured in RPMI 1640 medium supplemented with filtered 10% fetal bovine serum (FBS), penicillin, and streptomycin (100 U/ml of each) at 37℃ in a humidified atmosphere of 5% CO2. CdSe/ZnS quantum dots (Lumidot™) were purchased from Sigma-Aldrich (St. Louis MO, USA).
Freshly drawn, heparinized blood from a donor was used to isolate lymphocytes by Ficoll-Hypaque (Amersham Biosciences, Uppsala, Sweden) density gradient centrifugation at 400 ×g. After extraction from the gradient interface, lymphocytes were washed twice with phosphate buffered saline (PBS) and resuspended in RPMI 1640 medium (Gibco, Invitrogen, Carlsbad, CA, USA) containing 10% FBS and 100 U/ml each of penicillin and streptomycin. Lymphocytes were stimulated with 1% phytohemagglutinin (PHA; Gibco, Invitrogen, CA, USA) and cultured in a humidified atmosphere at 37℃ and 5% CO2.
Preparation and characterization of CdSe/ZnS QDs. CdSe/ZnS QDs dispersed in toluene were supplied by Sigma-Aldrich (St. Louis, MO, USA). In this study, we used the same CdSe/ZnS QD nanoparticles as in our previous study (2). Briefly, we removed the toluene using rotary evaporation (Rotavapor R-210; Buchi Laboratory Equipment, Zurich, Switzerland) and suspended the QDs in distilled water, which was sonicated for 30 min at 4℃ before each experiment. Dynamic light scattering (DLS), an indirect method of particle size determination, was measured at room temperature with an ALV/CGS-3 Compact Goninometer System (Hessen, Germany) equipped with a He-Ne laser operating at 632.8 nm. Transmission electron microscopy (TEM; 100CX; JEOL, Tokyo, Japan) was used to determine the size and shape of the CdSe/ZnS QDs. Absorption peaks of the CdSe/ZnS QDs were measured using an ultraviolet-visible (UV-Vis) spectrophotometer (V- 650; Jasco, Tokyo, Japan), and photoluminescence spectra were detected with a fluorescence spectrophotometer (F- 4500; Hitachi, Tokyo, Japan). The QD surface charge was measured in distilled water using a zeta potential analyzer (Zeta plus; Brookhaven Instruments Corp., Holtsville, NY, USA).
Curcumin preparation. Curcumin (Sigma-Aldrich, St. Louis, MO, USA) was diluted in dimethyl sulfoxide (DMSO) and added to cells at 0.5, 1, 2, or 4 mg/ml. The final concentration of DMSO was 1%, which had no effect on cell viability (Fig. 1).
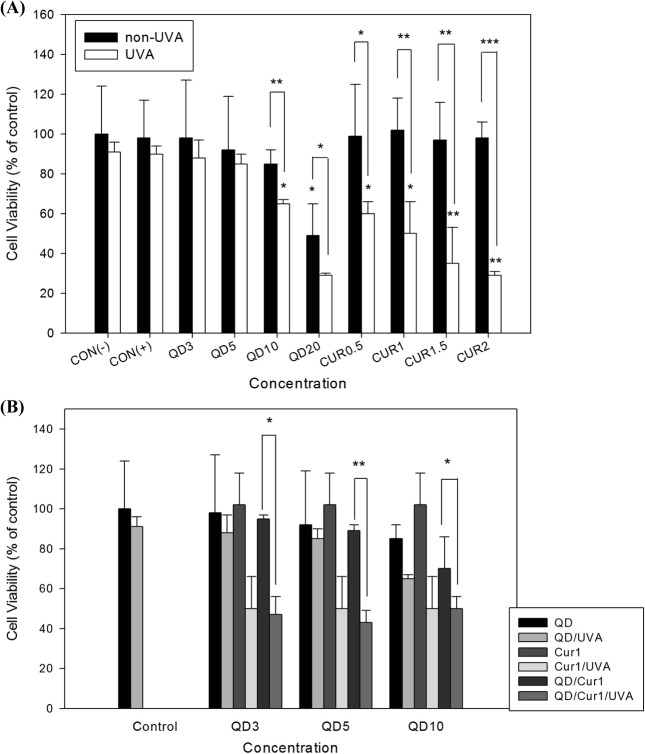
Fig. 1. HL-60 cell viability following treatment with QDs and/or curcumin in the presence or absence of UVA irradiation. Cells were incubated in a 96-well microplate for 24 h, treated with 3, 5, 10, or 20 μg/ml QDs or 0.5, 1, 1.5, or 2 μg/ml curcumin, respectively (A) and co-exposed to 3, 5, or 10 μg/ml QDs and 1 μg/ml curcumin (B). Untreated control, CON(−); negative control (treated with DMSO), CON(+). Cell viability was determined by the WST assay. Data are shown as percentages of the control, and error bars represent standard deviations (S.D.) of duplicate experiments. Results were evaluated statistically by oneway ANOVA and Student’s t-test. * p < 0.05, ** p < 0.01, *** p < 0.001.
Cytotoxicity assay. Cytotoxic effects induced by CdSe/ ZnS QDs and/or curcumin in HL-60 cells and normal human lymphocytes were evaluated by the Water Soluble Tetrazolium (WST) assay (Daeil Lab Service Co. Ltd., Seoul, Korea). Two types of cells were seeded in each 96-well plate at a density of 1 × 106 cells/100 μl, pretreated with curcumin for 1 hr, and incubated for 24 hr at 37℃ in a humidified atmosphere of 5% CO2. Sequentially, the cells were treated with CdSe/ZnS QDs at various concentrations with/without UVA (2 J/cm2) irradiation. A Bio-Link BLX irradiation system (Vilber-Lourmat, Marne-la-Vallee Cdex 1, France) equipped with five 8-W UV lamps (UV-A Vilber-Lourmat T- 8L with peak irradiance at 365) was used. The WST solution (100 μl) was added to each well and the plate was incubated for 4 h. The absorbance at 492 nm was measured using a microplate reader (Tecan, Männedorf, Switzerland).
Single-cell gel electrophoresis assay. Single-cell gel electrophoresis was performed as described by Singh et al. (16). HL-60 cells and human normal lymphocytes were treated with curcumin and/or CdSe/ZnS QDs for 1 hr and 3 hr, respectively, followed by UVA (2 J/cm2) exposure. After two PBS washes, the cells were maintained at 4℃ to prevent DNA repair. Slides were prepared according to the method of Singh (16). Briefly, images of 60 cells randomly selected from each sample were analyzed using a Komet 5.5 image analysis system (Kinetic Imaging Ltd., Nottingham, UK). The Olive tail moment (OTM) of each cell was measured under a fluorescence microscope (Nikon, Tokyo, Japan) equipped with a 515 to 560 nm excitation filter and 590 nm barrier filter.
Cytokinesis-block micronucleus assay. The cytokinesis- block micronucleus (MN) assay was performed as described by Fenech (17). HL-60 cells were cultured in RPMI 1640 medium supplemented with 10% FBS, incubated with curcumin and/or CdSe/ZnS QDs for 1 hr and 3 hr, respectively, and then irradiated with UVA. Cytochalasin B (4 μg/ml; Sigma-Aldrich, St. Louis, MO, USA) was added 20 hr after the start of culture, and the incubation was continued for an additional 28 hr.
Human peripheral whole blood (1 ml) was cultured in 9 ml of RPMI 1640 media supplemented with 10% FBS and treated with curcumin and/or CdSe/ZnS QDs under the same conditions as the HL-60 cells. After 44-h incubation, cytochalasin B was added to the culture and the cells were incubated for another 28 hr.
After a total incubation period of 48 hr for HL-60 cells and 72 hr for whole blood lymphocytes, the cells were harvested, treated with hypotonic 0.075 M KCl solution for 1 min, and washed twice with fixative solution (methanol : acetic acid, 3 : 1). Air-dried cell preparations were stained with Giemsa solution (5%). In total, 1,000 binucleated cells with well-preserved cytoplasm were scored according to standard criteria (18). In addition, cytokinesis-block proliferation index (CBPI) was evaluated in 500 cells from each sample in HL-60 cells and normal lymphocytes to determine the percentage of bi- and multi-nucleated cells.
Measurement of ROS. Intracellular ROS generation was assayed using 5-(and-6)-carboxy-2', 7'-dichlorofluorescein diacetate (DCFDA; Molecular Probes, Eugene, OR, USA). HL-60 cells and isolated lymphocytes were treated with curcumin for 1 hr and QDs for 3 hr, and then irradiated with UVA. Thereafter the medium was discarded through centrifugation, and fresh culture medium containing 20 μM DCFDA was added in the dark. The cells were incubated for 20 min at 37℃ and fluorescence distribution was monitored by flow cytometry (FACS Caliber, Becton-Dickinson, CA, USA).
Measurement of apoptosis. Apoptosis induction by curcumin and/or CdSe/ZnS QDs with/without UVA irradiation was determined by propidium iodide (PI) staining and flow cytometry. HL-60 cells and normal lymphocytes exposed to curcumin and/or CdSe/ZnS QDs were collected, washed twice with PBS, and fixed with 70% cold aqueous ethanol for 24 hr and then stored at −20℃ for at least 24 hr. Cells were washed twice with PBS and cell pellets were stained with PI solution containing RNase A (10 mg/ml) and PI (10 mg/ml) in PBS. The cell suspension was incubated in the dark at room temperature for 30 min, and DNA content was assayed by flow cytometry (FACS Caliber, Becton- Dickinson, CA, USA).
Statistical analysis. The effects of curcumin and/or CdSe/ZnS QDs with or without UVA irradiation on DNA were evaluated using non-parametric Kruskal-Wallis oneway analysis of variance (ANOVA) and the Mann-Whitney U test. One-way ANOVA and Student’s t-tests were applied to analyze cell viability. A difference of p < 0.05 was considered to indicate statistical significance. Data are shown as means ± standard deviation (S.D.).
RESULTS
Physicochemical characterization of QDs. As we reported previously, the average size of core-shell type CdSe/ZnS QDs as determined by DLS (particle size, 3.3 nm; λem, 530 nm) was 105 ± 15.55 nm, indicating that QD particles aggregate in an aqueous environment. The CdSe/ZnS QD UV-Vis absorption spectrum showed broad continuous absorption at wavelengths ranging from UV to visible. The zeta potential measurement in distilled water showed a ζ potential of + 40.58 ± 7.97 mV, confirming the colloidal stability of CdSe/ZnS QDs (2).
Effect of curcumin on HL-60 cell viability following QD treatment under UVA irradiation. WST assays were performed in HL-60 cells to assess the cytotoxicity of curcumin and/or CdSe/ZnS QDs with/without UVA irradiation. HL-60 cells were preincubated with curcumin for 1 hr and then treated with QDs, followed by irradiation with UVA light. An overall concentration-dependent decrease in cell viability was observed in cells treated with QDs and/or curcumin. The effect of curcumin as a photosensitizer was more significant than that of CdSe/ZnS QDs. Significant reductions in cell viability of 60%, 50%, 35%, and 29% were observed for curcumin treatments of 0.5, 1, 1.5, and 2 μg/ml, respectively, under UVA irradiation as compared with the control group, including the DMSO-treated negative control (Fig. 1A). In CdSe/ZnS QD-treated cells, 88%, 85%, 65%, and 29% viabilities were observed following treatment with QD concentrations of 3, 5, 10, and 20 μg/ml, respectively. Thus, combination treatment with CdSe/ZnS QDs (5 μg/ml) and curcumin (1 μg/ml) under UVA irradiation was highly effective at killing HL-60 cancer cells (Fig. 1B).
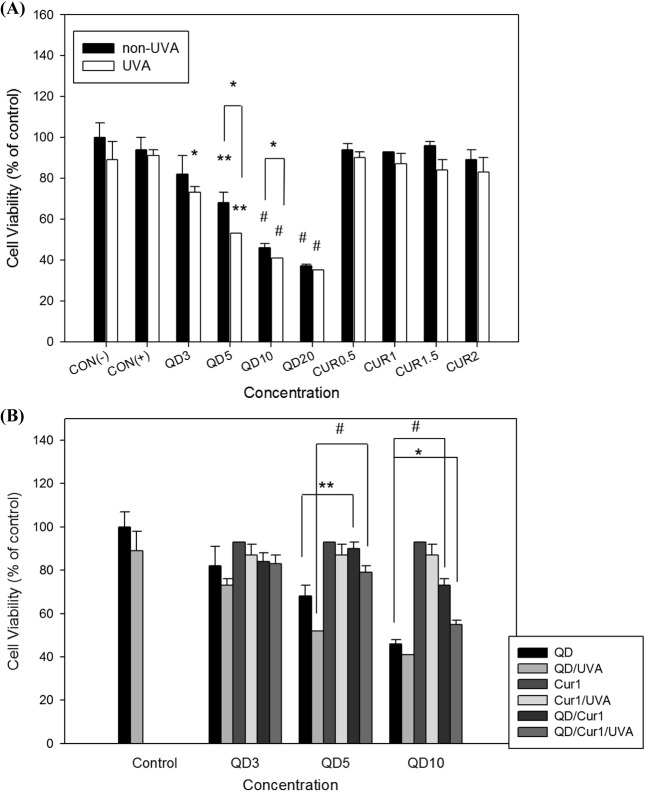
Effect of curcumin on normal lymphocyte viability following QD treatment under UVA irradiation. To assess the effect of curcumin on QD-induced cytotoxicity in normal lymphocytes isolated from whole blood, the WST assay was used. CdSe/ZnS QD treatment decreased the viability of normal lymphocytes in a concentration-dependent manner, but curcumin with/without UVA irradiation had a similar effect on normal lymphocytes as in control groups (Fig. 2A). Curcumin treatment protected against CdSe/ZnS QD-induced cytotoxicity in normal lymphocytes, as seen in Fig. 2B. CdSe/ZnS QDs (5 and 10 μg/ml) in combination with 1 μg/ml curcumin increased cell viabilities by 90% and 73% respectively, which were reduced by UVA irradiation to 79% and 55%, as compared with CdSe/ZnS QDs alone (68% and 46%, respectively). Although UVA irradiation decreased cell viability somewhat, the protective effect of curcumin on normal lymphocytes was statistically significant (p < 0.05).
Fig. 2. Cultured human normal lymphocyte cell viability following treatment with QDs and/or curcumin in the presence or absence of UVA irradiation. Cells were incubated in a 96-well microplate for 24 h, treated with 3, 5, 10, 20 μg/ml QDs or 0.5, 1, 1.5, 2 μg/ml curcumin, respectively (A) and co-exposed to 3, 5, 10 μg/ml QDs and 1 μg/ml curcumin (B). CON(−), untreated control; CON(+), negative control (treated with DMSO). Cell viability was determined by the WST assay. Data are shown as percentages of the control and error bars represent standard deviations of duplicate experiments. Results were assessed statistically by one-way ANOVA and Student’s t-test. * p < 0.05, ** p < 0.01, # p < 0.001.
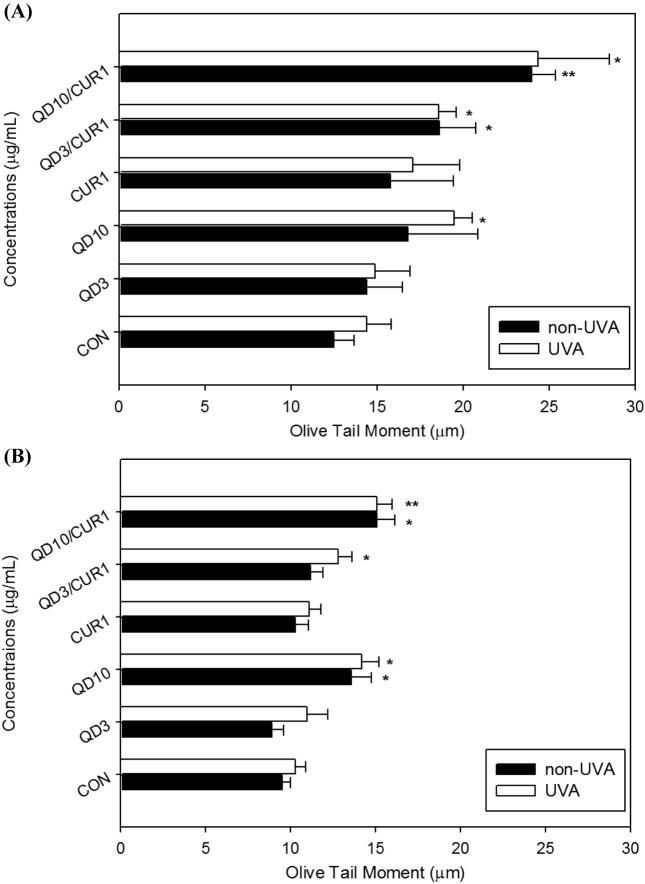
Effect of curcumin on CdSe/ZnS QD-induced DNA damage as indicated by the comet assay. We evaluated genotoxicity induced by curcumin and/or CdSe/ZnS QDs with/without UVA irradiation in HL-60 cells and normal human lymphocytes using the comet assay, in which the extent of DNA damage is represented by OTM (Fig. 3). Significant DNA damage was evident in HL-60 cells treated with 10 μg/ml QDs plus UVA irradiation (OTM: 19.45 ± 1.05), cells treated with 3 μg/ml QDs and curcumin (OTM: 18.62 ± 2.11), and cells treated with 3 μg/ ml QDs and curcumin plus UVA (OTM: 18.59 ± 1.02) as compared with the control group. Combination treatment with curcumin and QDs induced statistically significant single-stranded DNA damage in HL-60 leukemia cells (Fig. 3A).
Fig. 3. Single-stranded DNA breaks in HL-60 cells treated with QDs and/or curcumin with/without UVA irradiation. Single-stranded DNA breakage was evaluated by Olive tail movements (OTM) using the comet assay in HL-60 cells (A), and human normal lymphocytes (B). Values represent means±standard deviation (S.D.). Results were analyzed statistically by Kruskal-Wallis and Mann- Whitney’s U tests. * p < 0.05, ** p < 0.01 compared to the control.
OTM in normal lymphocytes treated with 3 μg/ml CdSe/ ZnS QDs and 1 μg/ml curcumin was slightly increased as compared with the control group (OTM: 11.2 ± 0.72 for 3 μg/ml QDs plus curcumin and 9.6 ± 0.51 for control). In this study, no protective effect of curcumin against QDinduced DNA single strand breaks was found in normal lymphocytes (Fig. 3B).
Effect of curcumin on CdSe/ZnS QD-induced DNA damage by micronucleus assay. We also performed MN assays to assess the genotoxic effects of curcumin on CdSe/ ZnS QDs with/without UVA irradiation (Table 1). The MN frequency in HL-60 control cells was 2.5 ± 0.7 per 1,000 binucleated cells; the frequencies in cells treated with QDs at 3 and 10 μg/ml were 8.0 ± 1.4 and 17.0 ± 7.0, respectively. The frequencies of MN formation were 3.5 ± 0.7, 12.5 ± 2.1, and 19.5 ± 9.0 per 1,000 binucleated cells for control, 3, and 10 μg/ml QDs, respectively, with UVA irradiation. The MN frequencies induced by combination treatment with curcumin and QDs were 19.5 ± 9.0 and 31.5 ± 17.7 for 3 μg/ml QDs/1 μg/ml curcumin and 10 μg/ml QDs/1 μg/ml curcumin, respectively. HL-60 cell MN frequencies were increased markedly when UVA irradiation was combined with QD and curcumin treatment. MN frequencies induced by combination treatment with 3 μg/ml QDs or 10 μg/ml QDs and 1 μg/ml curcumin plus UVA irradiation were 24 ± 8.0 and 42.5 ± 9.2, respectively (Table 1). The reduction in QD-induced MN formation in normal human lymphocytes with curcumin (reduction from 24.0 ± 5.6 to 22.0 ± 6.3) indicates that curcumin was more efficacious in protecting against genotoxicity, although not significantly so (Table 1). Finally, the combination treatment with curcumin and QDs increased MNs formation and decreased CBPI of cells.
Table 1.
Induction of micronuclei (MN) in HL-60 cells and human normal lymphocytes treated with QDs and/or curcumin with/without UVA irradiation
| HL-60 cells | Human normal lymphocytes | ||||
|---|---|---|---|---|---|
| Concentration (μg/mL) | Frequency of MNs/1000BNCs | CBPI | Concentration (μg/mL) | Frequency of MNs/1000BNCs | CBPI |
| Control | 2.5 ± 0.7 | 1.63 | Control | 8.0 ± 3.0 | 1.39 |
| Control/UVA | 3.5 ± 0.7 | 1.50 | Control/UVA | 8.0 ± 1.0 | 1.31 |
| QD3 | 8.0 ± 1.4* | 1.58 | QD3 | 17.0 ± 2.0* | 1.32 |
| QD3/UVA | 12.5 ± 2.1* | 1.49 | QD3/UVA | 23.0 ± 6.0* | 1.26 |
| QD10 | 17.0 ± 7.0 | 1.58 | QD10 | 24.0 ± 6.0* | 1.21 |
| QD10/UVA | 19.5 ± 9.0 | 1.39 | QD10/UVA | 28.0 ± 4.0* | 1.17 |
| Cur1 | 12.0 ± 8.5 | 1.68 | Cur1 | 14.0 ± 6.0 | 1.31 |
| Cur1/UVA | 18.5 ± 7.8 | 1.44 | Cur1/UVA | 18.0 ± 4.0* | 1.26 |
| QD3/Cur1 | 19.5 ± 9.0 | 1.49 | QD3/Cur1 | 22.0 ± 4.0* | 1.31 |
| QD3/Cur1/UVA | 24.0 ± 8.0* | 1.38 | QD3/Cur1/UVA | 22.0 ± 4.0* | 1.30 |
| QD10/Cur1 | 31.5 ± 17.7 | 1.47 | QD10/Cur1 | 22.0 ± 6.0 | 1.30 |
| QD10/Cur1/UVA | 42.5 ± 9.2* | 1.42 | QD10/Cur1/UVA | 26.0 ± 7.0* | 1.24 |
Micronuclei induction was measured by cytokinesis block micronucleus assay. MN, micronuclei; BNCs, binucleated cells; CBPI, cytokinesisblock proliferation index. Values represent means ± standard deviation (S.D.). Data were analyzed statistically using Kruskal-Wallis tests and Mann-Whitney U-tests. * p<0.05 compared to the control.
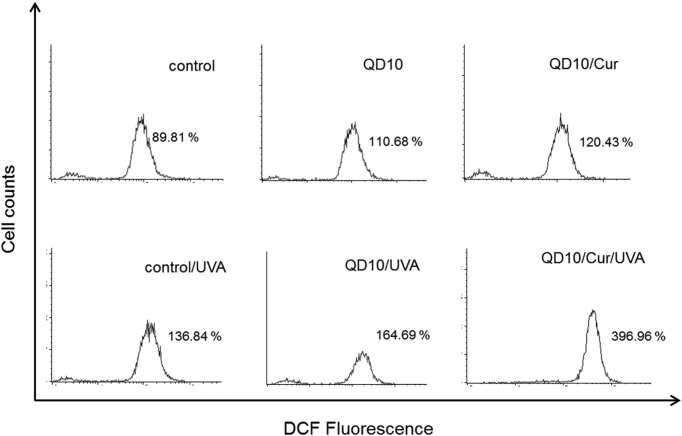
Effect of curcumin on CdSe/ZnS QD-induced intracellular ROS generation. ROS generation was determined by DCF fluorescence in HL-60 cells and detected by flow cytometry. Intracellular ROS content increased slightly in control cells treated with UVA, CdSe/ZnS QD treatment alone, QD treatment with UVA exposure, and QD plus curcumin co-treatment; ROS formation significantly increased in in cells irradiated with UVA in combination with QD and curcumin co-treatment. ROS generation in cells treated with 10 μg/ml QDs and curcumin plus UVA irradiation was increased fourfold compared to the control group (Fig. 4). These results indicate that UVA irradiation enhances ROS generation in HL-60 cells treated with a combination of CdSe/ZnS QDs and curcumin.
Fig. 4. Effect of curcumin on reactive oxygen species (ROS) generation in QD-treated HL-60 cells under UVA irradiation. HL-60 cells were pretreated with curcumin for 1 h, prior to treatment with QDs. After 3 h, the cells were irradiated with UVA light (2 J/cm2) and ROS generation was assayed by flow cytometry. Significant differences were observed following treatment with 10 μg/ml QDs and 1 μg/ml curcumin plus UVA irradiation as compared with the other treatment groups.
Assessment of apoptosis. We next conducted apoptosis assays to investigate the mode of CdSe/ZnS QD and/or curcumin-induced cell death in the presence or absence of UVA irradiation. HL-60 cells treated with QDs alone and/or exposed to UVA, co-treatment of QDs with curcumin and/ or UVA exposure, and co-treatment of QDs with curcumin and/or UVA exposure for 24 hr resulted in a concentrationdependent increase in apoptotic cell death, which increased to ~32.73% following QD and curcumin treatment plus UVA irradiation, as compared to 6.98% for the untreated control. Apoptotic cell death occurred in ~16.97% of normal human lymphocytes following treatment with the same concentrations as in the HL-60 cells, as compared to 11.88% in the untreated controls (Table 2). These results clearly indicate that the QD and curcumin combined treatment enhances apoptosis in cancer cells, whereas the combination has a protective effect in normal human lymphocytes.
Table 2.
The percentages of apoptotic cells measured using flow cytometry in HL-60 cells and normal lymphocytes
| Concentrations (μg/mL) | HL-60 cells | Normal lymphocytes |
|---|---|---|
| Control | 6.98 ± 6.75 | 11.88 ± 7.77 |
| QD5 | 7.21 ± 3.09 | 14.66 ± 11.81 |
| QD10 | 8.05 ± 2.10 | 18.27 ± 9.44 |
| Control/UVA | 7.96 ± 6.26 | 13.81 ± 9.90 |
| QD5/UVA | 8.76 ± 7.26 | 15.99 ± 11.53 |
| QD10/UVA | 14.62 ± 3.10 | 18.72 ± 15.66 |
| Cur1 | 5.74 ± 4.00 | 13.79 ± 5.15 |
| QD5/Cur1 | 7.01 ± 2.30 | 9.92 ± 5.95 |
| QD10/Cur1 | 13.04 ± 8.99 | 11.02 ± 5.94 |
| Cur1/UVA | 14.52 ± 13.34 | 15.39 ± 8.53 |
| QD5/Cur1/UVA | 22.60 ± 19.71 | 15.95 ± 9.55 |
| QD10/Cur1/UVA | 32.73 ± 23.20 | 16.97 ± 11.38 |
Data represent the mean ± S.D obtained from two independent experiments. Results are statistically analyzed by one-way ANOVA test, but not significant in either group (p>0.05).
DISCUSSION
Semiconductor nanocrystal QDs, which display unique optical and electrical properties, are being used in many biological applications. Although QDs have numerous novel physicochemical characteristics that make them one of the most advantageous tools in nanomedicine, many concerns as to human health have been raised due to their toxicity (19). In a previous study, we established that CdSe/ZnS QDs induced cyto-/genotoxicity through DNA damage, ROS induction, and activation of the apoptotic signaling pathway in A549 cells; we also proposed that they were a potential photosensitizer in photodynamic therapy (2). The limitation of QD use due to their toxicity requires more effective cancer treatments that are selectively targeted toward cancer cells without damaging healthy tissue. Here, we used a combined QD and curcumin treatment, two potential photosensitizers, to destroy cancer cells and protect normal cells. Curcumin, a natural compound, is widely known as an antioxidant, anticlastogen, anti-inflammatory and antimutagenic agent that protects normal tissues (10). The aim of this study was to identify a more effective cancer treatment that does not damage normal cells by evaluating the selective effect of CdSe/ZnS QDs and curcumin on HL-60 cells and normal human lymphocytes.
Upon comparing cell viability and apoptosis profiles between HL-60 cells and normal human lymphocytes, we observed distinct sensitivities to the QD and curcumin combined treatment, indicating the selective role of curcumin. Cell death and apoptosis were enhanced by curcumin combined with QDs plus UVA exposure in HL-60 cells (Fig. 1 and Table 2). Unlike HL-60 cells, curcumin had a protective effect against QD-induced toxicity on normal lymphocytes (Fig. 2 and Table 2). The selective effect of curcumin on the two cell types was also observed for ROS generation. ROS generation was markedly higher in HL-60 cells following co-treatment of QDs and curcumin with UVA exposure (Fig 4), while curcumin attenuated the QD-induced ROS in normal lymphocytes (data not shown). This is consistent with previous reports that curcumin has antioxidant activity but acts as a pro-oxidant under certain conditions (20-22). ROS caused by QD and curcumin exposure under UVA irradiation clearly increased single- and double-stranded DNA breaks as compared to QD treatment alone, QD/UVA co-exposure, curcumin treatment alone, curcumin/UVA coexposure, or QD and curcumin co-treatment in HL-60 cells (Fig. 3A and Table 1). Although the protective effect of curcumin was not statistically significant, the micronuclei frequency was lower in QD and curcumin-co-treated than in QD-treated cultured normal lymphocytes. Our results suggest that curcumin did not produce cyto-/genotoxicity, but protected normal lymphocytes from QD-induced cell death, apoptosis, and ROS generation regardless of UVA irradiation. In contrast to the normal lymphocytes, curcumin enhanced apoptotic cell death, ROS generation, and DNA damage induced by QDs in HL-60 cancer cells. Furthermore, the reduced cell viability was precipitated by UVA irradiation with QDs and curcumin in leukemia cells. The effect of curcumin in inducing apoptosis in cancer cells as opposed to normal cells is dependent upon various mechanisms, such as down-regulation of cyclin D and G2-M phase arrest in the cell proliferation pathway, a reduction in glutathione (GSH) levels resulting from ROS generation, caspase pathway activation, and higher uptake of curcumin by cancer cells (23-26).
Taken together, our data suggest that QD and curcumin co-exposure with UVA irradiation dramatically increased apoptotic cell death in HL-60 cells, and that this effect was mediated by ROS generation. Conversely, curcumin acted as an antioxidant in normal lymphocytes. The results of the present study indicate a novel treatment strategy for leukemia using a combination of CdSe/ZnS QDs and curcumin.
Acknowledgments
This study was supported by Basic Science Research Program (2012-0002464) through the National Research Foundation of Korea (NRF) and Brain Korea 21 (2012) program funded by the Ministry of Education, Science and Technology.
References
- 1.Hauck T.S., Anderson R.E., Fischer H.C., Newbigging S., Chan W.C. In vivo quantum-dot toxicity assessment. Small. (2010);6:138–144. doi: 10.1002/smll.200900626. [DOI] [PubMed] [Google Scholar]
- 2.Choi Y.J., Kim Y.J., Lee J.W., Lee Y., Lim Y.B., Chung H.W. Cyto-/genotoxic effect of CdSe/ZnS quantum dots in human lung adenocarcinoma cells for potential photodynamic UV therapy applications. J. Nanosci. Nanotechnol. (2012);12:2160–2168. doi: 10.1166/jnn.2012.5781. [DOI] [PubMed] [Google Scholar]
- 3.Chibli H., Carlini L., Park S., Dimitrijevic N.M., Nadeau J.L. Cytotoxicity of InP/ZnS quantum dots related to reactive oxygen species generation. Nanoscale. (2011);3:2552–2559. doi: 10.1039/c1nr10131e. [DOI] [PubMed] [Google Scholar]
- 4.Morosini V., Bastogne T., Frochot C., Schneider R., François A., Guillemin F., Barberi-Heyob M. Quantum dot-folic acid conjugates as potential photosensitizers in photodynamic therapy of cancer. Photochem. Photobiol. Sci. (2011);10:842–851. doi: 10.1039/c0pp00380h. [DOI] [PubMed] [Google Scholar]
- 5.Pons T., Pic E., Lequeux N., Cassette E., Bezdetnaya L., Guillemin F., Marchal F., Dubertret B. Cadmiumfree CuInS2/ZnS quantum dots for sentinel lymph node imaging with reduced toxicity. ACS Nano. (2010);4:2531–2538. doi: 10.1021/nn901421v. [DOI] [PubMed] [Google Scholar]
- 6.Valizaden A., Mikaeili H., Samiel M., Farkhani S.M., Zarghami N., Kouhi M., Akbarzadeh A., Davaran S. Quantum dots: synthesis, bioapplications, and toxicity. Nanoscale Res. Lett. (2012);7:480. doi: 10.1186/1556-276X-7-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Byrne S.J., Williams Y., Davies A., Corr S.A., Rakovich A., Gun’ko Y.K., Rakovich Y.P., Donegan J.F., Volkov Y. “Jelly dots”: synthesis and cytotoxicity studies of CdTe quantum dot-gelatin nanocomposites. Small. (2007);3:1152–1156. doi: 10.1002/smll.200700090. [DOI] [PubMed] [Google Scholar]
- 8.Mazumder S., Dey R., Mitra M.K., Mukherjee S., Das G.C. Review: Biofunctionalized quantum dots in biology and medicine. J. Nanomater. (2009) Article ID 815734, 17. [Google Scholar]
- 9.Dovigo L.N., Pavarina A.C., Ribeiro A.P., Brunetti I.L., Costa C.A., Jacomassi D.P., Bagnato V.S., Kurachi C. Investigation of the photodynamic effects of curcumin against Candida albicans. Photochem. Photobiol. (2011);87:895–903. doi: 10.1111/j.1751-1097.2011.00937.x. [DOI] [PubMed] [Google Scholar]
- 10.Zhang B.Y., Shi Y.Q., Chen X., Dai J., Jiang Z.F., Li N., Zhang Z.B. Protective effect of curcumin against formaldehyde-induced genotoxicity in A549 cell lines. J. Appl. Toxicol. (2012) doi: 10.1002/jat.2814. [DOI] [PubMed] [Google Scholar]
- 11.Ayli E.E., Dugas-Breit S., Li W., Marshall C., Zhao L., Meulener M., Griffin T., Gelfand J.M., Seykora J.T. Curcuminoids activate p38 MAP kinases and promote UVB-dependent signaling in keratinocytes. Exp. Dermatol. (2010);19:493–500. doi: 10.1111/j.1600-0625.2010.01081.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang H.C., Lin C.J., Liu W.J., Jiang R.R., Jiang Z.F. Dual effects of curcumin on neuronal oxidative stress in the presence of Cu(II). Food Chem. Toxicol. (2011);49:1578–1583. doi: 10.1016/j.fct.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 13.Wilken R., Veena M.S., Wang M.B., Srivatsan E.S. Curcumin: A review of anti-cancer properties and therapeutic activity in head and neck squamous cell carcinoma. Mol. Cancer. (2011);10:10–12. doi: 10.1186/1476-4598-10-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahn J.C., Kang J.W., Shin J.I., Chung P.S. Combination treatment with photodynamic therapy and curcumin induces mitochondria-dependent apoptosis in AMC-HN3 cells. Int. J. Oncol. (2012);41:2184–2190. doi: 10.3892/ijo.2012.1661. [DOI] [PubMed] [Google Scholar]
- 15.Park K., Lee J.H. Photosensitizer effect of curcumin on UVB-irradiated HaCaT cells through activation of caspase pathways. Oncol. Rep. (2007);17:537–540. [PubMed] [Google Scholar]
- 16.Singh N.P., McCoy M.T., Tice R.R., Schneider E.L. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp. Cell. Res. (1988);175:184–191. doi: 10.1016/0014-4827(88)90265-0. [DOI] [PubMed] [Google Scholar]
- 17.Fenech M. The cytokinesis-block micronucleus technique: a detailed description of the method and its application to genotoxicity studies in human populations. Mutat. Res. (1993);285:35–44. doi: 10.1016/0027-5107(93)90049-l. [DOI] [PubMed] [Google Scholar]
- 18.Fenech M. The in vitro micronucleus technique. Mutat. Res. (2000);455:81–95. doi: 10.1016/s0027-5107(00)00065-8. [DOI] [PubMed] [Google Scholar]
- 19.Clift M.J., Stone V. Quantum dots: an insight and perspective of their biological interaction and how this relates to their relevance for clinical use. Theranostics. (2012);2:668–680. doi: 10.7150/thno.4545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahsan H., Hadi S.M. Strand scission in DNA induced by curcumin in the presence of Cu(II). Cancer Lett. (1998);124:23–30. doi: 10.1016/s0304-3835(97)00442-4. [DOI] [PubMed] [Google Scholar]
- 21.Mahakunakorn P., Tohda M., Murakami Y., Matsumoto K., Watanabe H., Vajaragupta O. Cytoprotective and cytotoxic effects of curcumin: dual action on H2O2-induced oxidative cell damage in NG108-15 cells. Biol. Pharm. Bull. (2003);26:725–728. doi: 10.1248/bpb.26.725. [DOI] [PubMed] [Google Scholar]
- 22.Cao J., Jia L., Zhou H.M., Liu Y., Zhong L.F. Mitochondrial and nuclear DNA damage induced by curcumin in human hepatoma G2 cells. Toxicol. Sci. (2006);91:476–483. doi: 10.1093/toxsci/kfj153. [DOI] [PubMed] [Google Scholar]
- 23.Choudhuri T., Pal S., Das T., Sa G. Curcumin selectively induces apoptosis in deregulated cyclin D1- expressed cells at G2 phase of cell cycle in a p53-dependent manner. J. Biol. Chem. (2005);280:20059–20068. doi: 10.1074/jbc.M410670200. [DOI] [PubMed] [Google Scholar]
- 24.Syng-Ai C., Kumari A.L., Khar A. Effect of curcumin on normal and tumor cells: Role of glutathione and bcl-2. Mol. Cancer Ther. (2004);3:1101–1108. [PubMed] [Google Scholar]
- 25.Kunwar A., Barik A., Mishra B., Rathinasamy K., Pandey R., Priyadarsini K.I. Quantitative cellular uptake, localization and cytotoxicity of curcumin in normal and tumor cells. Biochim. Biophys. Acta. (2008);1780:673–679. doi: 10.1016/j.bbagen.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 26.Ravindran J., Prasad S., Aggarwal B.B. Curcumin and cancer cells: how many ways can curry kill tumor cells selectively? AAPS J. (2009);11:495–510. doi: 10.1208/s12248-009-9128-x. [DOI] [PMC free article] [PubMed] [Google Scholar]