Abstract
Armeniacae semen (AS) has been considered a toxic herb in the Korean medicine as it contains hydrogen cyanide and amygdalin, especially in its endocarp. Therefore, prebrewed AS that is devoid of endocarp has been traditionally used. In the present study, amygdalin content of the prebrewed AS was significantly lower (2.73 ± 0.32 μg/ml; p < 0.01) than the content in the extract that contained the endocarps (28.50 ± 6.71 μg/ml); amygdalin content corresponded to 10% of the extract in the present study. Because of single oral dose toxicity of prebrewed AS according to the recommendation of Korea Food and Drug Administration Guidelines (2009-116, 2009), which was based on single oral dose toxicity study of prebrewed AS, mortality due to toxic principles was significantly reduced. In this study, 2,000 mg/kg of prebrewed AS led to death of 1 female rat and 1 male rat at the end of 2 hr of administration. Based on these results, the 50% lethal dose in both male and female rats was determined to be 9279.5 mg/kg. Seizure, loss of locomotion, and increases in respiration and heart rate were observed as prebrewed AS treatment-related toxicological signs; these signs were restrictedly manifested in the prebrewed AS (2,000 mg/kg)-treated rats. In addition, no changes were observed in body weight, organ weight, gross features, and histopathological parameters with 2,000 mg/kg of AS in both male and female rats. These findings serve as direct evidence that amygdalin in AS is the toxic principle, which can be reduced by the traditional prebrewing method involving the exclusion of endocarp.
Keywords: Prebrewed Armeniacae Semen, Single oral dose toxicity, Rat, Amygdalin, Histopathology
INTRODUCTION
Natural products are gaining space and importance in the pharmaceutical industry as well as inspiring the search for new potential sources of bioactive molecules (1,2). Herbs, medicinal plants and crude drug substances are considered to be a potential source of antioxidants to combat various diseases (3). As increase of the concern in the functional food and well being in life, the demands and consumption of functional food originated from natural sources are increased (4). However, the toxicological aspects about these natural origin-functional foods have been neglected because of the reasons that they have been used as various purposes for long times (5). Therefore, it is considered that more detail and systemic toxicological studies should be tested for control the abuse and potential toxicities even if they have been used as traditional folk medicine.
Armeniacae Semen (AS) is a dried seed part of Prunus armeniaca Linne var. ansu Maximowicz or Prunus mandshurica Koehne var. glabra Nakai (Family: Rosaceae), and has been traditionally used in Korean medicine for treating constipation and various respiratory diseases (6-8). Active components of AS or crude AS extract itself have been shown various favorable pharmacological effects as inhibitory effects on asthma (6), allergy (9), mutagenicity (10), cancer (11,12) and inflammations (13). However, AS has been regarded as a representative toxic herb in Korean medicine, it contains source of hydrogen cyanide, toxic amygdalin, especially in endocarp, which can be induced life-threaten respiratory disorders (14-16). Therefore, endocarp excluded prebrewed AS has been traditionally used as an ingredients of Korean traditional herbal formulas to reduce the general toxicity related to amygdalin (7,8). In the previous study, 50% lethal dose (LD50) and approximate lethal dose (ALD) of yield 19.0% endocarp included crude AS aqueous extracts, to the best of our knowledge, after single oral administration in rats are known as 741.95 mg/kg and 500~1,000 mg/kg, respectively (16). However, there are no detailed toxicological assessments of prebrewed endocarp excluded AS has been reported even the rodent single oral dose toxicity test.
In the present study, the changes in the amygdalin contents were observed in endocarp excluded prebrewed AS as compared with endocarp included AS by UPLC (Ultra Performance Liquid Chromatography) method, and single oral dose toxicity test of prebrewed AS aqueous extracts were conducted in rats according to the Korea Food and Drug Administration (KFDA) Guidelines (17) to obtain the primary safety information about prebrewed AS and further clarifies their safety for clinical use.
MATERIALS AND METHODS
Preparation of AS aqueous extracts. Aqueous endocarp included AS (yield = 19.60%) and endocarp excluded prebrewed AS (yield = 22.00%) were prepared by routine methods using rotary vacuum evaporator (Buchi Rotavapor R-144, Switzerland) and programmable freeze dryer (Freezone 1; Labconco Corp., MO, USA) from dried seed part of Prunus armeniaca Linne var. ansu Maximowicz produced around Hebei, China, which were purchased from Omniherb (Korea) after confirmation of the morphology under microscopy. The voucher specimens documenting this purchase were deposited in the herbarium of the Medical Research center for Globalization of Herbal Formulation, Daegu Haany University. In the present study, prepared herbs were boiled at 80℃, 3 hrs and then, evaporated and lysophilized. Powders of AS extracts including or excluding endocarp are light brown powder, and all extracts were stored in a refrigerator at −20℃ to protect from light and degeneration until use. They were well soluble up to 200 mg/ml concentration levels in distilled water used as vehicle as clear light brown solution.
Measurement of amygdalin contents in AS extracts by UPLC.
Chromatography conditions: The UPLC system (Waters, USA), equipped with a pump Waters ACQUITYTM ultra performance LC system (Waters, USA) and a Waters ACQUITYTM photodiode array detector (PDA), was used for analysis. The Empower Data System was used for recording of the output signal of the detector. A Waters ACQUITYTM BEH C18 column (1.7 μm, 2.1 × 100 mm) was used for separation. The mobile phase was composed of 0.1% formic acid water and 0.1% formic acid acetonitrile (Sigma, USA) with the gradient elution system at a flow rate of 0.4 ml/min. The injection volume was 2 μl. The detection UV wavelength was set at 254 nm. The column temperature was set at room temperature.
Preparation of standard solutions and sample: Standard stock solutions of amygdalin (Wako, Japan) were prepared by dissolving at a concentration of 1000 μg/ml in 10 ml of methanol. Working standard solutions were made by diluting the standard stock solution with methanol. Standard stock solutions and working solutions were stored at 4℃. For the preparation of the sample, the extract of AS or prebrewed AS was weighed and dissolved in methanol at a concentration of 10 mg/ml. Before UPLC analysis, the sample preparation was filtered through a 0.22 μm filter. Amygdalin contents were expressed as mean ± SD of three independent measurements as μg/ml, in this experiment.
Experimental animals and administration of prebrewed AS extracts: Each of twenty female and male Spraque-Dawley rats (6-wk old upon receipt, SLC, Japan) were used after acclimatization for 7 days. Animals were allocated five per polycarbonate cage in a temperature (20~25℃) and humidity (45~50%) controlled room. Light : dark cycle was 12 hrs : 12 hrs, and food (Samyang, Korea) and water were supplied free to access. All animals were overnight fasted before administration and terminal necropsy. Animals were marked by picric acid. This study was carried out according to the guidelines of the Animal Ethical Committee, The University of Daegu Haany University (Korea). Prebrewed AS have been used as Korean medicine as an ingredient of herbal formula for long times and no revealed toxicological data was available, the highest dosage level was selected as 2,000 mg/kg according to the recommended by KFDA Guidelines (2005) and Organization for Economic Co-Operation and Development (OECD) Guidelines (#423) (18), the limited dosages, and 1,000 and 500 mg/kg was selected using common ratio 2. In addition, a vehicle control group was added. Animal was once orally administered using a sonde attached to a syringe of 3 ml after overnight fasting (about 18 hr, water was not restricted), at a dosage volume of 10 ml/kg. Food and water were restricted further for about 3 hrs after end of administration.
Abnormal behavior, clinical sign and body weight: All abnormal clinical signs and behaviors were recorded before and after dosing at least twice a day based on the functional observational battery test (19,20). Body weights were measured on the day of administration (Day 0) prior to treatment, 1, 2, 7, 13 and 14 days after dosing, respectively.
Necropsy: All unscheduled died animals were grossly observed immediately after finding them and all survived animals were subjected to terminal necropsy. Animals were asphyxiated by carbon dioxide and gross necropsy was performed in all animals at Day 14 after overnight fasting (about 18 hrs, water was not restricted).
Organ weight measurements and sampling: The absolute organ weight was measured and then relative organ weight (% for body weight) was calculated. The following organs were collected for histopathological observation: lung, heart, thymus, left kidney, left adrenal gland, spleen, left testis or ovary, liver, splenic lobe of pancreas, prostate, urinary bladder, left epididymis or total uterus and left submandibular lymph node.
Histopathology: Samples were fixed in 10% neutral buffered formalin. After 18 hrs of fixation, paraffin embedding was conducted and 4 μm sections were prepared by routine histological methods. Representative sections of each specified organs were stained with hematoxylin-eosin for light microscopical examination.
Statistical analyses: Multiple comparison tests for different dose groups were conducted. Variance homogeneity was examined using the Levene test (21). If the Levene test indicated no significant deviations from variance homogeneity, the obtained data were analyzed by one way ANOVA test followed by Scheffe test to determine which pairs of group comparison were significantly different. In case of significant deviations from variance homogeneity were observed at Levene test, a non-parametric comparison test, the Mann-Whitney U (MW) test was conducted to determine the specific pairs of group comparison, which are significantly different (22). LD50 and 95% confidence limits were calculated by Probit method. Statistical analyses were conducted using SPSS for Windows (Release 14.0K, SPSS Inc., USA) and a p-value of less than 0.05 was considered to be a significant difference. In addition, degree of clinical signs, gross and histopathological findings were subdivided into 3 degrees: 3+ Severe, 2+ moderate, 1+ slight, according to the previous reports (5,23,24).
RESULTS
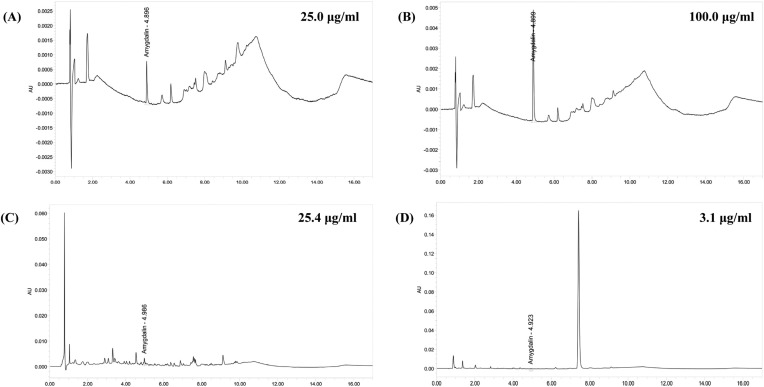
Analysis of amygdalin. Determination of amygdalin in AS extracts was established by the use of the UPLC system. Contents of the amygdalin were calculated from the calibration curve of the standards (Fig. 1). Validation of the method verified its reliability and stability. Use of the method resulted in successive separation of amygdalin in AS samples. Significant (p < 0.01) decreases of amygdalin contents in excluded prebrewed AS extracts (2.73 ± 0.32 μg/ml) were determined as compared with endocarp included AS extracts (28.50 ± 6.71 μg/ml) in this study.
Fig. 1. UPLC chromatogram of three marker compounds in AS. UPLC chromatogram of commercial standard amygdalin (A, B). UPLC chromatogram of amygdalin in endocarp included AS (C) and prebrewed endocarp excluded AS (D). The chromatograms were obtained at 254 nm. AS, Armeniacae Semen aqueous extracts.
Mortalities. No unscheduled or prebrewed AS extractstreatment related mortalities were detected in all dose levels tested in this study, except for one female and male rats treated with prebrewed AS extracts 2,000 mg/kg; they were died within 2 hrs after the treatment (Table 1).
Table 1.
Mortality, clinical signs, gross and histopathological findings of animals exposed with prebrewed AS in the single dose toxicity study
| Groups | Male vehicle control | Prebrewed AS treated male rats (mg/kg) | Female vehicle control | Prebrewed AS treated female rats (mg/kg) | ||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| 2,000 | 1,000 | 500 | 2,000 | 1,000 | 500 | |||
|
| ||||||||
| Mortalitya) | 0/5 | 1/5 | 0/5 | 0/5 | 0/5 | 1/5 | 0/5 | 0/5 |
| Clinical signs | ||||||||
| Seizure | 0/5 | 2/5 | 0/5 | 0/5 | 0/5 | 1/5 | 0/5 | 0/5 |
| Loss of locomotionb) | 0/5 | 5/5 | 0/5 | 0/5 | 0/5 | 3/5 | 0/5 | 0/5 |
| Gross findings | ||||||||
| Lung focal congestion | 1/5 | 1/4 | 1/5 | 1/5 | 1/5 | 1/4 | 0/5 | 1/5 |
| Thymus focal congestion | 1/5 | 0/4 | 0/5 | 0/5 | 0/5 | 0/4 | 0/5 | 0/5 |
| Spleen atrophy | 1/5 | 0/4 | 1/5 | 0/5 | 1/5 | 0/4 | 0/5 | 1/5 |
| Lymph node hypertrophyc) | 1/5 | 1/4 | 2/5 | 2/5 | 2/5 | 1/4 | 3/5 | 1/5 |
| Uterus edema | 1/5 | 2/4 | 0/5 | 1/5 | ||||
| Histopathological findings | ||||||||
| Lung focal congestion | 1/5 | 1/4 | 1/5 | 1/5 | 1/5 | 1/4 | 0/5 | 1/5 |
| Tymus cDEd) | 0/5 | 0/4 | 0/5 | 0/5 | 1/5 | 0/4 | 0/5 | 0/5 |
| Spleen wDEe) | 2/5 | 1/4 | 1/5 | 0/5 | 1/5 | 0/4 | 1/5 | 1/5 |
| Liver focal inflammation | 2/5 | 1/4 | 1/5 | 0/5 | 2/5 | 2/4 | 1/5 | 2/5 |
| Lymph node HPcf) | 2/5 | 1/4 | 2/5 | 3/5 | 3/5 | 2/4 | 3/5 | 2/5 |
Values were observed animals/total observed animals.
AS = Armeniacae Semen aqueous extracts.
a)Each of one female and male rat treated with 2,000mg/kg of prebrewed AS was died, within 2 hr after end of treatment, respectively.
b)Loss of locomotion with increases of heart beat and respiration.
c)Submandibular lymph node.
d)Cortex lymphoid cell decreases.
e)White pulp lymphoid cell decreases.
f)Diffused hyperplasia of lymphoid cells.
Clinical signs. In this study, various degrees of loss of locomotion and increases of heart beat and respirations were detected in all five (5/5; 100%) male and three (3/5; 60%) female rats treated with 2,000 mg/kg, at to the treatment day, respectively. In addition, one (1/5; 20%) female and two (2/5; 40%) male rats treated with prebrewed AS extracts 2,000 mg/kg additionally showed slight to moderate seizure at treatment day. These abnormal prebrewed AS treatment related clinical signs were disappeared within 2 hrs after the end of the treatment, if they were survived in this experiment (Table 1).
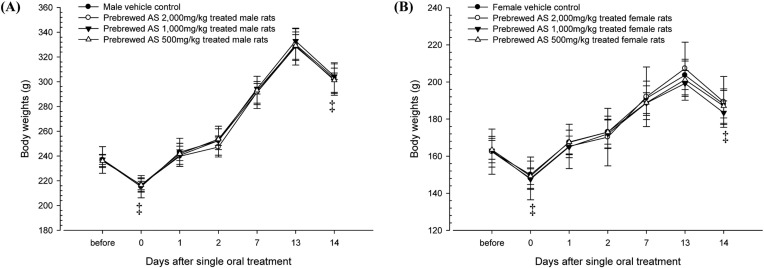
Changes in body weights. No significant changes in body weight were detected as compared with equal genders of vehicle control rats in all dose levels tested in this experiment (Fig. 2).
Fig. 2. Changes on the body weights during 14 days of observation in female (A) and male (B) rats after single oral treatment of prebrewed AS extracts. No significant changes on body weights were detected in all prebrewed AS extract treated rats as compared with equal genders of vehicle control rats, respectively. Values are expressed as mean ± SD of five rats except for 2,000mg/kg treated rats, which were four rats. AS, Armeniacae Semen aqueous extracts. †‘, all rats were overnight fasted; before means 1 day before administration. 0 means the day of administration.
Changes in the organ weight. No meaningful changes in the organ weights were observed in all survived prebrewed AS extracts treated female and male rats to the termination, as compared with each equal gender of vehicle control (Table 2 and 3).
Table 2.
Absolute organ weights of animals exposed with prebrewed AS in the single dose toxicity study
| Groups | Male vehicle control | Prebrewed AS treated male rats (mg/kg) | Female vehicle control | Prebrewed AS treated female rats (mg/kg) | ||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| 2,000 | 1,000 | 500 | 2,000 | 1,000 | 500 | |||
|
| ||||||||
| Organ weights (g) | ||||||||
| Lung | 1.26 ± 0.09 | 1.26 ± 0.09 | 1.28 ± 0.05 | 1.20 ± 0.02 | 0.96 ± 0.07 | 0.98 ± 0.08 | 0.95 ± 0.06 | 0.98 ± 0.03 |
| Heart | 0.93 ± 0.04 | 0.97 ± 0.03 | 0.97 ± 0.04 | 0.93 ± 0.05 | 0.67 ± 0.04 | 0.68 ± 0.06 | 0.66 ± 0.04 | 0.66 ± 0.04 |
| Thymus | 0.53 ± 0.07 | 0.51 ± 0.05 | 0.50 ± 0.10 | 0.49 ± 0.04 | 0.40 ± 0.03 | 0.43 ± 0.04 | 0.44 ± 0.02 | 0.44 ± 0.05 |
| Kidney (left) | 0.97 ± 0.04 | 0.97 ± 0.03 | 0.94 ± 0.03 | 0.92 ± 0.04 | 0.65 ± 0.05 | 0.61 ± 0.03 | 0.60 ± 0.07 | 0.60 ± 0.05 |
| Adrenal gland (left) | 0.04 ± 0.02 | 0.03 ± 0.01 | 0.04 ± 0.01 | 0.03 ± 0.02 | 0.04 ± 0.07 | 0.04 ± 0.01 | 0.04 ± 0.01 | 0.04 ± 0.01 |
| Spleen | 0.62 ± 0.05 | 0.58 ± 0.01 | 0.62 ± 0.05 | 0.63 ± 0.03 | 0.40 ± 0.04 | 0.39 ± 0.02 | 0.41 ± 0.05 | 0.41 ± 0.05 |
| Testis/Ovary (left) | 1.64 ± 0.07 | 1.60 ± 0.05 | 1.61 ± 0.05 | 1.55 ± 0.09 | 0.08 ± 0.01 | 0.07 ± 0.02 | 0.07 ± 0.01 | 0.07 ± 0.01 |
| Liver | 8.87 ± 0.40 | 8.84 ± 0.61 | 8.85 ± 0.18 | 8.80 ± 0.57 | 5.37 ± 0.30 | 5.44 ± 0.46 | 5.26 ± 0.29 | 5.24 ± 0.21 |
| Brain | 1.94 ± 0.07 | 1.92 ± 0.09 | 1.93 ± 0.05 | 1.95 ± 0.06 | 1.80 ± 0.04 | 1.86 ± 0.07 | 1.81 ± 0.05 | 1.76 ± 0.05 |
| Lymph node (left)a) | 0.06 ± 0.02 | 0.05 ± 0.07 | 0.06 ± 0.01 | 0.06 ± 0.01 | 0.05±0.02 | 0.04 ± 0.01 | 0.05 ± 0.02 | 0.05 ± 0.01 |
| Epididymis (left) | 0.37 ± 0.03 | 0.36 ± 0.05 | 0.42 ± 0.06 | 0.37 ± 0.02 | ||||
| Pancreas | 0.64 ± 0.06 | 0.65 ± 0.10 | 0.65 ± 0.08 | 0.61 ± 0.08 | 0.45 ± 0.04 | 0.42 ± 0.06 | 0.44 ± 0.04 | 0.43 ± 0.03 |
| Urinary bladder | 0.35 ± 0.04 | 0.42 ± 0.09 | 0.37 ± 0.05 | 0.38 ± 0.07 | 0.08 ± 0.01 | 0.08 ± 0.01 | 0.07 ± 0.01 | 0.08 ± 0.01 |
| Prostate/Uterus | 0.10 ± 0.02 | 0.09 ± 0.01 | 0.10 ± 0.01 | 0.09 ± 0.01 | 0.33 ± 0.08 | 0.40 ± 0.08 | 0.32 ± 0.03 | 0.40 ± 0.19 |
Values are expressed as mean ± S.D. of five rats except for 2,000mg/kg treated rats, which were four rats.
AS = Armeniacae Semen aqueous extracts.
a)Submandibular lymph node.
Table 3.
Relative organ weights of animals exposed with prebrewed AS in the single dose toxicity study
| Groups | Male vehicle control | Prebrewed AS treated male rats (mg/kg) | Female vehicle control | Prebrewed AS treated female rats (mg/kg) | ||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| 2,000 | 1,000 | 500 | 2,000 | 1,000 | 500 | |||
|
| ||||||||
| Organ weights (% of body weight) | ||||||||
| Lung | 0.42 ± 0.02 | 0.42 ± 0.02 | 0.42 ± 0.02 | 0.40 ± 0.02 | 0.51 ± 0.02 | 0.52 ± 0.02 | 0.52 ± 0.02 | 0.52 ± 0.02 |
| Heart | 0.31 ± 0.02 | 0.32 ± 0.01 | 0.32 ± 0.01 | 0.31 ± 0.01 | 0.36 ± 0.02 | 0.36 ± 0.02 | 0.36 ± 0.02 | 0.35 ± 0.02 |
| Thymus | 0.18 ± 0.02 | 0.18 ± 0.02 | 0.17 ± 0.03 | 0.16 ± 0.02 | 0.21 ± 0.02 | 0.23 ± 0.03 | 0.24 ± 0.01 | 0.23 ± 0.02 |
| Kidney (left) | 0.32 ± 0.02 | 0.32 ± 0.01 | 0.31 ± 0.01 | 0.30 ± 0.01 | 0.34 ± 0.02 | 0.33 ± 0.03 | 0.33 ± 0.03 | 0.32 ± 0.02 |
| Adrenal gland (left) | 0.01 ± 0.01 | 0.01 ± 0.01 | 0.01 ± 0.01 | 0.01 ± 0.01 | 0.02 ± 0.01 | 0.02 ± 0.01 | 0.02 ± 0.01 | 0.02 ± 0.01 |
| Spleen | 0.20 ± 0.01 | 0.19 ± 0.01 | 0.21 ± 0.02 | 0.21 ± 0.01 | 0.21 ± 0.02 | 0.20 ± 0.01 | 0.22 ± 0.02 | 0.22 ± 0.02 |
| Testis/Ovary (left) | 0.54 ± 0.02 | 0.53 ± 0.01 | 0.53 ± 0.02 | 0.52 ± 0.04 | 0.04 ± 0.01 | 0.04 ± 0.01 | 0.04 ± 0.01 | 0.04 ± 0.01 |
| Liver | 2.94 ± 0.09 | 2.92 ± 0.12 | 2.91 ± 0.08 | 2.92 ± 0.14 | 2.86 ± 0.08 | 2.87 ± 0.14 | 2.87 ± 0.11 | 2.80 ± 0.10 |
| Brain | 0.64 ± 0.02 | 0.63 ± 0.02 | 0.63 ± 0.02 | 0.65 ± 0.03 | 0.96 ± 0.05 | 0.98 ± 0.07 | 0.99 ± 0.04 | 0.95 ± 0.05 |
| Lymph node (left)a) | 0.19 ± 0.01 | 0.02 ± 0.01 | 0.02 ± 0.01 | 0.02 ± 0.01 | 0.03 ± 0.01 | 0.02 ± 0.01 | 0.02 ± 0.01 | 0.03 ± 0.01 |
| Epididymis (left) | 0.12 ± 0.01 | 0.12 ± 0.02 | 0.14 ± 0.02 | 0.12 ± 0.01 | ||||
| Pancreas | 0.21 ± 0.01 | 0.21 ± 0.03 | 0.21 ± 0.03 | 0.20 ± 0.03 | 0.24 ± 0.02 | 0.22 ± 0.02 | 0.24 ± 0.02 | 0.23 ± 0.01 |
| Urinary bladder | 0.32 ± 0.01 | 0.03 ± 0.01 | 0.03 ± 0.01 | 0.03 ± 0.01 | 0.04 ± 0.01 | 0.04 ± 0.01 | 0.04 ± 0.01 | 0.04 ± 0.01 |
| Prostate/Uterus | 0.12 ± 0.02 | 0.14 ± 0.03 | 0.12 ± 0.02 | 0.13 ± 0.02 | 0.18 ± 0.05 | 0.22 ± 0.06 | 0.17 ± 0.02 | 0.21 ± 0.09 |
Values are expressed as mean ± S.D. of five rats except for 2,000mg/kg treated rats, which were four rats.
AS = Armeniacae Semen aqueous extracts.
a)Submandibular lymph node.
Necropsy findings. No prebrewed AS extracts-treatment related changes in the gross findings were observed, except for some sporadic findings such as slight congestion spots of lung and thymus, spleen atrophy, hypertrophy of submandibular lymph node and edematous changes of uterus. They were sporadically detected throughout all experimental groups tested in the present including both gender of vehicle control (Table 1).
Histopathological findings. Some sporadic findings such as slight hypertrophy of lung alveolus wall with focal hemorrhage, decreases of lymphoid cells in the cortex of thymus, decreases of white pulp lymphoid cells in spleen, focal inflammatory cell infiltration in the liver and diffused hyperplasia of lymphoid cells in the submandibular lymph node were sporadically detected throughout all experimental groups tested in the present study including both gender vehicle controls. No prebrewed AS extracts-treatment related changes in the histopathological findings were observed in this experiment (Table 1).
DISCUSSION
AS contains more than 3% amygdalin, 50% of fatty acid and 20~25% proteins (25) and also contains emulsion, setrone, estradiol-17β and prunasin (26). However, it most of the respiratory toxicity of AS are directly related to amygdalin (25). Since endocarp of AS contains source of hydrogen cyanide and amygdalin (14-16), endocarp excluded prebrewed AS has been traditionally used as an ingredients of traditional Korean medicine, mainly for treating constipation and various respiratory diseases (6-8). In the present study, we investigated the changes of amygdalin contents in endocarp excluded prebrewed AS as compared with endocarp included AS by UPLC, and single oral dose toxicity test of prebrewed AS aqueous extracts were conducted in rats as a part of the safety test. In order to observe LD50 and ALD, prebrewed AS aqueous extracts were single orally administered to female and male rats at dose levels of 2,000, 1,000 and 500 mg/kg according to the recommendation of KFDA Guidelines (17). The mortality and changes on body weight, clinical signs and gross observation were monitored during 14 days after oral administration of the extracts with organ weights and histopathology of principle organs.
Amygdalin contents in prebrewed AS extracts were significantly (p < 0.01) decreased as compared with endocarp included AS extracts; they were decreased as about 1/10 in endocarp excluded prebrewed AS extracts (2.73 ± 0.32 μg/ml) as compared with endocarp included AS extracts (28.50 ± 6.71 μg/ml) in this study. As results of single oral dose toxicity of prebrewed AS in this study, mortalities were restrictedly detected in one female and male rat treated with prebrewed AS at 2,000 mg/kg within 2 hrs after end of administration, therefore, LD50 and ALD in female and male rats were detected as 9279.50 mg/kg and 1,000~2,000 mg/kg, respectively. Seizure, loss of locomotion, increases of respirations and heart beats were also observed as prebrewed AS treatment related with toxicological clinical signs, which were restrictly detected in prebrewed AS 2,000 mg/kg treated rats. No body and organ weight changes, gross and histopathological observations were detected up to 2,000 mg/kg of prebrewed AS extracts in both female and male rats.
In KFDA Guidelines (17), the recommended highest dose of test materials were 2,000 mg/kg or the maximum solubility. They as a single oral dose toxicity test in rodents, they recommend below 20 ml/kg on the dosage volume in care of adequate solubility (27). In the present study, the highest dosage was selected of 2,000 mg/kg in a volume of 10 ml/kg, and 1,000 and 500 mg/kg are selected using common ratio 2. In addition, each female and male vehicle control groups were added. Test material was orally administered using distilled water as vehicle in the present study.
All survived animals showed body weight increases ranged in normal age-matched rats (28,29). In addition, no meaningful changes in the organ weights were observed in all prebrewed AS extracts treated female and male rats as compared with each matched gender of vehicle control.
Noticeably, amygdalin contents in prebrewed AS extracts were decreased as about 1/10 as compared with endocarp included AS extracts, and LD50 of prebrewed AS extracts were increased by 12.5 fold higher than those of previous endocarp included crude AS aqueous extracts (16) after single oral administration in rats. In the previous study, LD50 of yield 19.0% endocarp included crude AS aqueous extracts were detected as 741.95 mg/kg after single oral administration in rats (16). These findings are considered as direct evidences that general toxicity of AS would be reduced by traditional prebrewed method, excluding endocarp, along with the decreases of amygdalin contents.
Various degrees of loss of locomotion, increases of heart beat and respirations, seizure detected in some male and female rats treated with 2,000 mg/kg, were restricted to the treatment day, which were considered as treatment with toxicological signs related to amygdalin induced respiratory disorders; they have been detected as classic amygdalin intoxicated clinical signs (15,30,31). In addition, similar signs were also observed in the previous single oral dose toxicity test of endocarp included AS (16); clinical signs were observed in the lowest dosage group at 500 mg/kg, but they were restricted to the highest dosage at 2,000 mg/kg in this experiment and disappeared within 2 hrs after end of treatment, if they were survived, suggesting that amygdalin related clinical signs were reduced by traditional prebrewed methods.
The slight congestion spots of lung and thymus, spleen atrophy, hypertrophy of submandibular lymph node and edematous changes of uterus detected in the present study as gross aspects of histopathology, and hypertrophy of lung alveolus wall with focal hemorrhage, decreases of lymphoid cells in the cortex of thymus, decreases of white pulp lymphoid cells in spleen, focal inflammatory cell infiltration in the liver and diffused hyperplasia of lymphoid cells in the submandibular lymph node detected as histopathological findings were considered as accidental findings rather than toxicological signs related to the prebrewed AS extracts treatment because they were sporadically detected throughout experimental groups tested in the present study including both corresponding control group with vehicle. Especially, the edematous changes in uterus were considered as secondary changes from different physiological estrus cycles (32,33). In addition, most of them were also rarely but generally observed as sporadic accidental findings in rodent toxicity studies (5,23,24).
The results obtained in this study suggest that general toxicity of AS would be directly related to the amygdalin contents and the toxicity of AS was reduced by traditional prebrewed method, excluding endocarp as decrease of amygdalin content. However, AS extract should be carefully treated at clinical applications even after endocarp excluded because mortalities and amygdalin related clinical signs were also noticed in 2,000 mg/kg treated female and male rats. In this experiment, LD50 and ALD of prebrewed AS extracts in female and male rats were detected as 9279.50 mg/kg and 1,000~2,000 mg/kg.
References
- 1.Saravanan N., Rajasankar S., Nalini N. Antioxidant effect of 2-hydroxy-4-methoxy benzoic acid on ethanolinduced hepatotoxicity in rats. J. Pharm. Pharmacol. (2007);59:445–453. doi: 10.1211/jpp.59.3.0015. [DOI] [PubMed] [Google Scholar]
- 2.Devipriya N., Srinivasan M., Sudheer A.R., Menon V.P. Effect of ellagic acid, a natural polyphenol, on alcoholinduced prooxidant and antioxidant imbalance: a drug dose dependent study. Singapore Med. J. (2007);48:311–318. [PubMed] [Google Scholar]
- 3.Noh J.R., Kim Y.H., Gang G.T., Hwang J.H., Kim S.K., Ryu S.Y., Kim Y.S., Lee H.S., Lee C.H. Hepatoprotective effect of Platycodon grandiflorum against chronic ethanol-induced oxidative stress in C57BL/6 mice. Ann. Nutr. Metab. (2011);58:224–231. doi: 10.1159/000330117. [DOI] [PubMed] [Google Scholar]
- 4.Lee J.E., Kim H.J., Lee C.H., Lee K.C., Choi E.K., Chai H.Y., Yun Y.W., Kim D.J., Nam S.Y., Lee B.J., Ahn B.W. Four-week repeated-dose toxicity study on pinellia extract. Korean J. Lab. Anim. Sci. (2003);19:127–141. [Google Scholar]
- 5.Roh S.S., Ku S.K. Mouse single oral dose toxicity study of DHU001, a polyherbal formula. Toxicol. Res. (2010);26:53–59. doi: 10.5487/TR.2010.26.1.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Do J.S., Hwang J.K., Seo H.J., Woo W.H., Nam S.Y. Antiasthmatic activity and selective inhibition of type 2 helper T cell response by aqueous extract of semen armeniacae Amarum. Immunopharmacol. Immunotoxicol. (2006);28:213–225. doi: 10.1080/08923970600815253. [DOI] [PubMed] [Google Scholar]
- 7.Seo B.I., Park J.H. A philological study on poisoning of Armeniacae Amarum Semen. J. Jeahan Orient. Med. Acad. (2008);6:135–162. [Google Scholar]
- 8.Kim S.R., Lee J.W., Lim S.Y., Kim J.D. The study of literature review on poisoning and the pragmatic significance of the processing method of Armeniacae Semen to use in oriental medical prescription. Korean J. Orient. Med. Prescr. (2011);19:151–160. [Google Scholar]
- 9.Kim Y.S., Song C.H. Study on the anti-allergic effect of Armeniacae Semen herbal acupuncture solution. J. Meridian Acupoint. (2007);24:151–162. [Google Scholar]
- 10.Yamamoto K., Osaki Y., Kato T., Miyazaki T. [Antimutagenic substances in the Armeniacae Semen and Persicae semen]. Yakugaku Zasshi. (1992);112:934–939. doi: 10.1248/yakushi1947.112.12_934. [DOI] [PubMed] [Google Scholar]
- 11.Toriyama-Baba H., Iigo M., Asamoto M., Iwahori Y., Park C.B., Han B.S., Takasuka N., Kakizoe T., Ishikawa C., Yazawa K., Araki E., Tsuda H. Organotropic chemopreventive effects of n-3 unsaturated fatty acids in a rat multi-organ carcinogenesis model. Jpn. J. Cancer Res. (2001);92:1175–1183. doi: 10.1111/j.1349-7006.2001.tb02137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang H.K., Shin M.S., Yang H.Y., Lee J.W., Kim Y.S., Lee M.H., Kim J., Kim K.H., Kim C.J. Amygdalin induces apoptosis through regulation of Bax and Bcl-2 expressions in human DU145 and LNCaP prostate cancer cells. Biol. Pharm. Bull. (2006);29:1597–1602. doi: 10.1248/bpb.29.1597. [DOI] [PubMed] [Google Scholar]
- 13.Yang H.Y., Chang H.K., Lee J.W., Kim Y.S., Kim H., Lee M.H., Shin M.S., Ham D.H., Park H.K., Lee H., Kim C.J. Amygdalin suppresses lipopolysaccharide-induced expressions of cyclooxygenase-2 and inducible nitric oxide synthase in mouse BV2 microglial cells. Neurol. Res. (2007);29:S59–S64. doi: 10.1179/016164107X172248. [DOI] [PubMed] [Google Scholar]
- 14.Lee S.H., Rim H.J., Kim D.J., Kim K.S. A studies on the chemical composition of apricot seed. Korean J. Food Nutr. (1992);5:1–5. [Google Scholar]
- 15.Suchard J.R., Wallace K.L., Gerkin R.D. Acute cyanide toxicity caused by apricot kernel ingestion. Annu. Emerg. Med. (1998);32:742–744. doi: 10.1016/S0196-0644(98)70077-0. [DOI] [PubMed] [Google Scholar]
- 16.Kim S.R., Lee J.W., Lim S.Y., Jung Y.S., Choi H.Y., Kim J.D. Rat single oral dose toxicity test of Armeniacae Semen (including endocarp). Korean J. Orient. Int. Med. (2012);33:145–159. [Google Scholar]
- 17.Korea Food and Drug Administration. Notification No. 2009-116. Testing Guidelines for Safety Evaluation of Drugs. (2009) issued by the Korea Food and Drug Administration on August 24, 2009.
- 18.Organization for Economic Co-Operation and Development. OECD guideline (423) for testing of chemicals, acute oral toxicity-acute toxic class method. (2001):1–14.
- 19.Irwin S. Comprehensive observational assessment: Ia. A systemic, quantitative procedure for assessing the behavioral and physiological state of the mouse. Psychopharmacologia. (1968);13:222–257. doi: 10.1007/BF00401402. [DOI] [PubMed] [Google Scholar]
- 20.Dourish C.T., Greenshaw A.J., Dourish C.T. Effects of drugs on spontaneous motor activity in Experimental psychopharmacology. Humana Press; Clifton: (1987). pp. 325–334. [Google Scholar]
- 21.Levene A. Pathological factors influencing excision of tumours in the head and neck. Part I. Clin. Otalaryngol. Allied Sci. (1981);6:145–151. doi: 10.1111/j.1365-2273.1981.tb01800.x. [DOI] [PubMed] [Google Scholar]
- 22.Ludbrook J. Update: microcomputer statistics packages. A personal view. Clin. Exp. Pharmacol. Physiol. (1997);24:294–296. doi: 10.1111/j.1440-1681.1997.tb01823.x. [DOI] [PubMed] [Google Scholar]
- 23.Yoon H.S., Shin Y.K., Jung Y.M., Lee H.S., Ku S.K. Single oral dose toxicity test of low molecular weight fucoidan in rats. Biomol. Ther. (2009);17:325–331. doi: 10.4062/biomolther.2009.17.3.325. [DOI] [Google Scholar]
- 24.Yoon H.S., Shin Y.K., Lee S.H., Lee D.S., Jung Y.M., Lee H.S., Ku S.K. 14 days repeat oral dose toxicity of low molecular weight fucoidan in rats. Biomol. Ther. (2010);18:111–121. doi: 10.4062/biomolther.2010.18.1.111. [DOI] [Google Scholar]
- 25.Zhu Y.P. Chinese Materia Medica Chemistry, Pharmacology and Applications. Harwood Academic Publisher; The Netherlands: (1998). pp. 496–498. [Google Scholar]
- 26.Nout M.J., Tuncel G., Brimer L. Microbial degradation of amygdalin of bitter apricot seeds (Prunus armeniaca). Int. J. Food Micribiol. (1995);24:407–412. doi: 10.1016/0168-1605(94)00115-M. [DOI] [PubMed] [Google Scholar]
- 27.Flecknell P. Laboratory Animal Anesthesia. 2nd Ed. Harcourt Brace & Company; New York: (1996). p. 269. [Google Scholar]
- 28.Fox J.G., Cohen B.J., Loew F.M. Laboratory animal medicine. Academic Press; Orlando Florida, USA: (1984). ISN 0122636201. [Google Scholar]
- 29.Tajima Y., Horiuchi S., et al. Biological reference data book on experimental animals. Soft Science; Tokyo: (1989). pp. 1–10. [Google Scholar]
- 30.Strugala G.J., Stahl R., Elsenhans B., Rauws A.G., Forth W. Small-intestinal transfer mechanism of prunasin, the primary metabolite of the cyanogenic glycoside amygdalin. Hum. Exp. Toxicol. (1995);14:895–901. doi: 10.1177/096032719501401107. [DOI] [PubMed] [Google Scholar]
- 31.O’Brien B., Quigg C., Leong T. Severe cyanide toxicity from ‘vitamin supplements’. Eur. J. Emerg. Med. (2005);12:257–258. doi: 10.1097/00063110-200510000-00014. [DOI] [PubMed] [Google Scholar]
- 32.Banks W.J., Banks W.J. Female reproductive system in Applied veterinary histology. Williams & Wilkins; Baltimore: (1986). pp. 506–526. [Google Scholar]
- 33.Pineda M.H., McDonald L.E., Pineda M.H. Female reproductive system in Veterinary endocrinology and reproduction. Lea & Febiger; Philadelphia: (1989). pp. 303–354. [Google Scholar]