Figure 7.
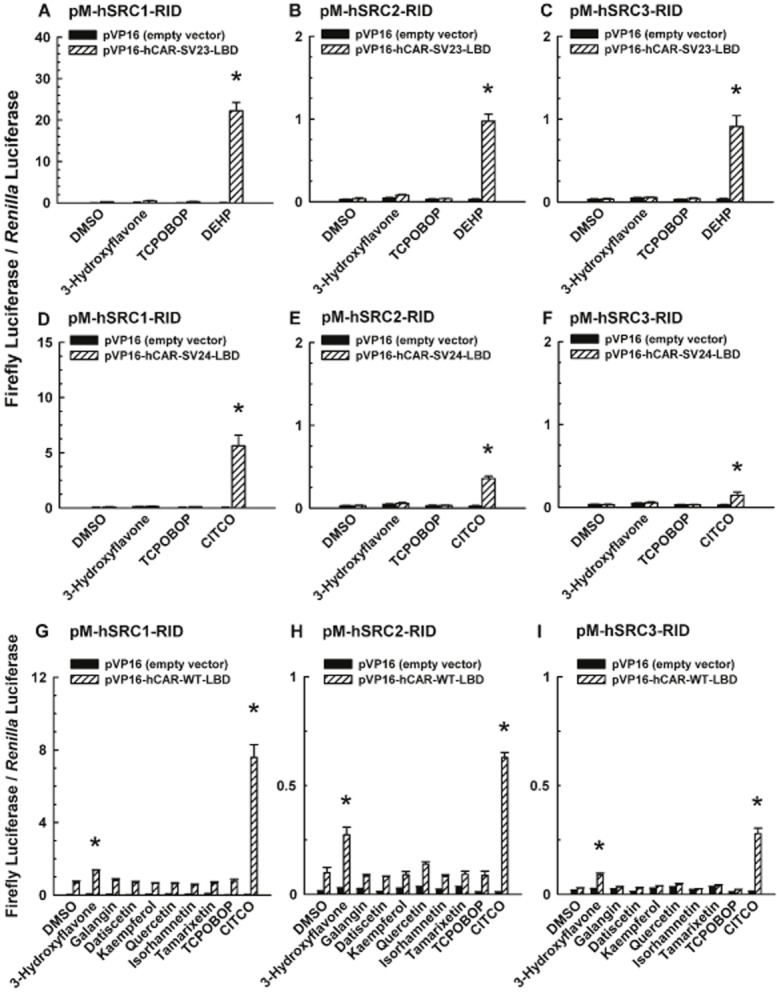
Effect of 3-hydroxyflavone and its structural analogues on coactivator recruitment to the LBD of hCAR-SV23, hCAR-SV24, and hCAR-WT. Cultured HepG2 cells were co-transfected with a receptor expression plasmid or the pVP16 empty vector, a coactivator expression plasmid, pCMV6-XL4-hRXRα (except for the hCAR-WT assay), pGL4.74[hRluc/TK] internal control plasmid, and pFR-luc reporter plasmid. The receptor expression plasmids were (A, B, C) pVP16-hCAR-SV23-LBD, (D, E, F) pVP16-hCAR-SV24-LBD, and (G, H, I) pVP16-hCAR-WT-LBD. The coactivator expression plasmids were (A, D, G) pM-hSRC1-RID, (B, E, H) pM-hSRC2-RID, and (C, F, I) pM-hSRC3-RID. Transfected cells were treated with DMSO (0.1% v/v; vehicle), a flavonol (3-hydroxyflavone, galangin, datiscetin, kaempferol, quercetin, isorhamnetin, or tamarixetin; each at 30 μM), TCPOBOP (0.25 μM), DEHP (10 μM), or CITCO (10 μM). In the hCAR-WT assay, androstanol (10 μM; inverse agonist of hCAR-WT) was added to each treatment group. Firefly luciferase and R. reniformis luciferase activities were quantified and normalized as described under Methods. Data are expressed as mean ± S.E.M. for three or four independent experiments performed in triplicate. *, significantly different from the same treatment group transfected with the empty vector and the vehicle-treated control group transfected with the same receptor expression plasmid (P < 0.05). Androstanol decreased hCAR-WT activity in the vehicle-treated control group by 43 ± 9%.
