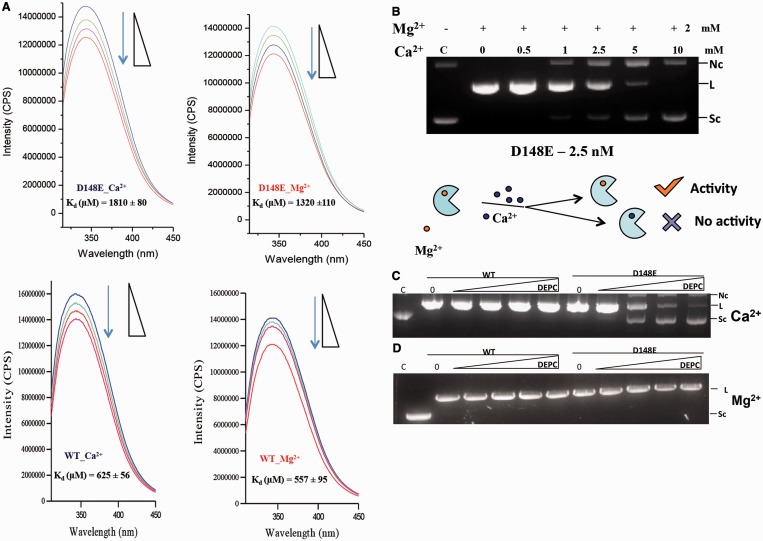
Figure 2.
Ca2+ ion binds to KpnI D148E but does not support DNA cleavage. (A) Fluorescence emission spectra of WT and mutant D148E in the presence of Ca2+ and Mg2+ with increasing amounts of CaCl2 (Blue) or MgCl2 (Red) (0–10 mM). Representative spectra and the calculated Kd values are shown. (B) Metal ion competition assay. Reactions were carried out by assaying Mg2+-mediated DNA cleavage activity in the presence of titrating Ca2+ ions. Reactions contained 2.5 nM D148E and competition was performed by pre-incubation of the enzyme in a buffer containing 10 mM Tris–HCl (pH 7.4), on ice with the indicated metal ions. The reactions were initiated by addition of pUC18 DNA (14 nM) and incubated at 37°C for 1 h. Enhanced solvent accessibility of His149 in the presence of Ca2+. KpnI WT or mutant D148E enzymes were pre-incubated with 5 mM of (C) CaCl2 or (D) MgCl2 and treated with increasing concentrations (25–100 μM) of DEPC. Residual activity was assayed by cleavage of pUC18 DNA (14 nM) in the presence of 2 mM MgCl2.