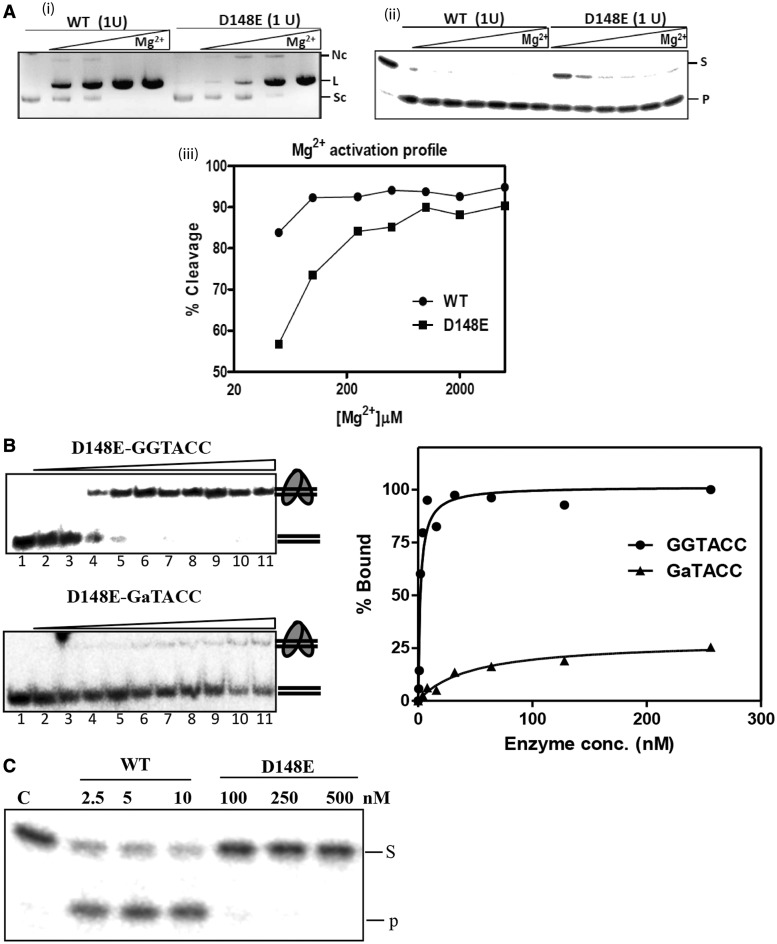
Figure 3.
Molecular basis for the high fidelity of variant D148E. (A) Mg2+-dependent DNA cleavage of mutant D148E. Different concentrations of metal ions (0–10 mM) were incubated with 1 unit of WT or mutant D148E in a buffer containing 10 mM Tris–HCl (pH 7.4), on ice for 5 min. DNA cleavage reaction was initiated by the addition of (i) pUC18 DNA (14 nM) or (ii) end labeled canonical oligonucleotide (10 nM) and incubated at 37°C for 1 h. (iii) Graphical representation of the Mg2+ activation profile of WT and D148E variant on the oligonucleotide substrate. Mutant D148E requires a Mg2+ concentration of 200 μM for the 90% digestion of the substrate, compared with 50 μM required by WT, showing that the decrease in binding affinity toward Mg2+ (Figure 2A) directly affects the cleavage activity of KpnI. (B) Canonical versus non-canonical discrimination by mutant D148E. EMSA was carried to determine the binding affinity of mutant D148E to end-labeled canonical (GGTACC) and non-canonical (GaTACC) substrates over a range of enzyme concentrations between 0 and 256 nM. The intensity of the shifted DNA band is expressed as percentage bound. (C) Mutant D148E does not cleave star substrate at binding concentrations. End-labeled oligonucleotide duplex containing the 5′-GaTACC-3′ sequence (10 nM) was incubated with WT (2.5–10 nM) or mutant D148E (100–500 nM) at 37°C for 30 min in the presence of 2 mM Mg2+. The cleavage products were analyzed on 12% urea-PAGE. Lane C contains the substrate DNA without the enzyme. S and P represent the substrate and product, respectively.