Figure 1.
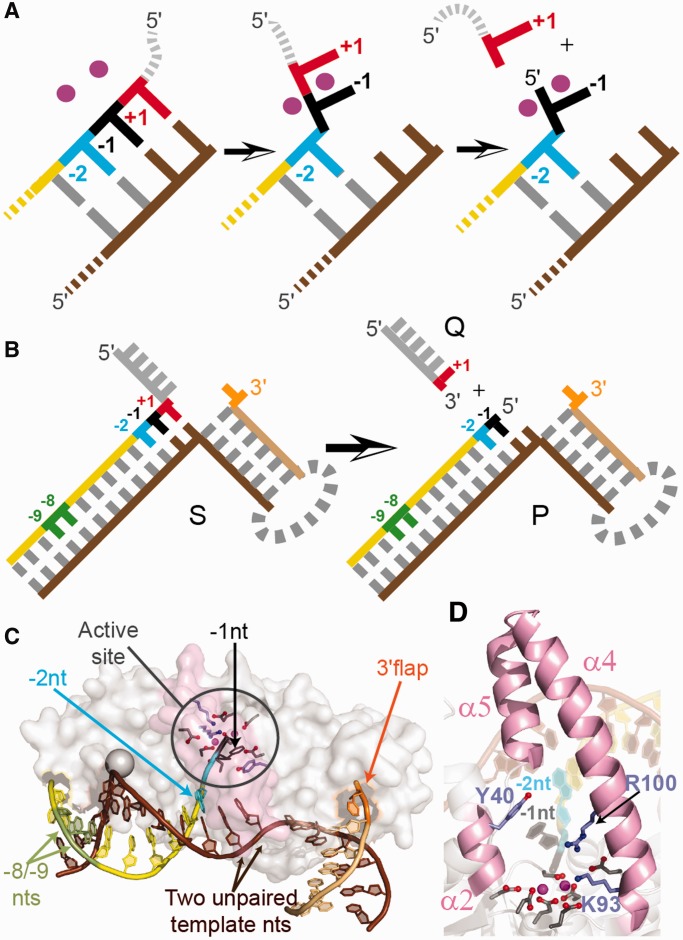
Schematic of proposed 5′-nuclease double nucleotide unpairing mechanism, reaction and supporting hFEN1-product structures. (A) Proposed unpairing of duplex ends that places the scissile phosphate diester in contact with active-site divalent ions (magenta). (B) The reaction of a static double-flap substrate (S) catalyzed by hFEN1 generates a 5′-phosphorylated product (P) and a single-stranded product (Q). Hydrolysis occurs between the +1 and −1 nucleotides (nts) as shown. (C) Structure of the hFEN1-product complex (3Q8K) highlighting two unpaired nucleotides of the template strand (brown), a single unpaired nt (−1 nt, black) of the CF strand (yellow) positioned on active-site metal ions (magenta), the 3′-flap nt (orange), the −2 nt (cyan), and −8 and −9 nts (green). (Product Q was not observed.) Protein is shown transparent with α4-α5 in pink. (D) The active site of hFEN1-P (colors as C). Side chains of superfamily conserved carboxylate residues (gray), K93, R100 and semi-conserved Y40 (light blue) are illustrated as sticks.