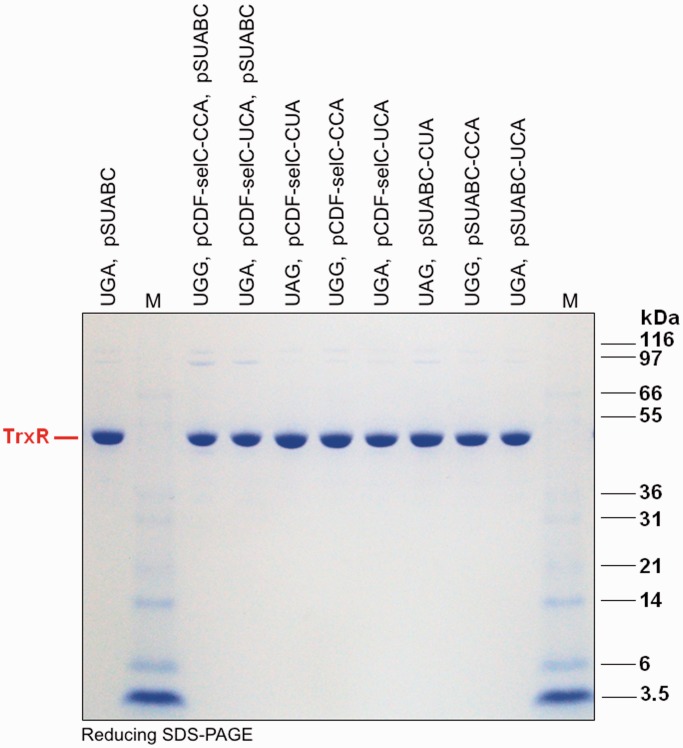
Figure 7.
Purity analysis of additional TrxR variants produced using alternative tRNASec variants. Using co-transformations with the pCDF-selC plasmid and/or pSUABC together with the pET-TRSTER TrxR1 expression plasmid in E. coli BL21 (DE3) gor− host strains, these TrxR1 were recombinantly expressed and further purified over 2′,5′-ADP SepharoseTM affinity chromatography followed by SuperdexTM G-200 gel filtration chromatography (GE Healthcare Life Sciences, Uppsala, Sweden). Sodium selenite was supplemented at 5 μM in the bacterial medium for all productions. The purified protein samples were here analyzed on reducing SDS–PAGE gels. ‘M’ stands for M12 protein standards (Life Technologies, USA), with size (kDa) indicated in the figure. The strong ≈ 55 kDa protein bands represents the resolved TrxR1 subunits, whereas the weak ≈110 kDa bands are traces of covalent TrxR1 dimers stable in reducing SDS–PAGE, as typically seen in analyses of this enzyme.