Abstract
A bone morphogenetic protein (BMP) obtained in solution by digestion of demineralized rabbit cortical bone matrix with bacterial collagenase retains its biologically active conformation in a neutral salt/ethylene glycol mixture. BMP may be insolubilized by coprecipitation with calcium phosphate and resolubilized by chemical extraction with a neutral salt in the same solvent mixture. Upon concanavalin A-Sepharose chromatography, BMP is bound by hydrophobic interaction and carbohydrate recognition and is recovered by elution with either alpha-methyl mannoside or ethylene glycol solvent mixture. Implants of both eluates and the extracts of the coprecipitate in double-walled diffusion chambers induce transmembrane bone morphogenesis. BMP is not species specific; rabbit BMP induces new bone formation in the rat. The present observations indicate that BMP is a glycoprotein.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashton B. A., Triffitt J. T., Herring G. M. Isolation and partial characterization of a glycoprotein from bovine cortical bone. Eur J Biochem. 1974 Jun 15;45(2):525–533. doi: 10.1111/j.1432-1033.1974.tb03577.x. [DOI] [PubMed] [Google Scholar]
- CESSI C., PILIEGO F. The determination of amino sugars in the presence of amino acids and glucose. Biochem J. 1960 Dec;77:508–510. doi: 10.1042/bj0770508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey M. W., Sulkowski E., Carter W. A. Binding of human fibroblast interferon to concanavalin A-agarose. Involvement of carbohydrate recognition and hydrophobic interaction. Biochemistry. 1976 Feb 10;15(3):704–713. doi: 10.1021/bi00648a039. [DOI] [PubMed] [Google Scholar]
- Firschein H. E., Shill J. P. The determination of total hydroxyproline in urine and bone extracts. Anal Biochem. 1966 Feb;14(2):296–304. doi: 10.1016/0003-2697(66)90140-0. [DOI] [PubMed] [Google Scholar]
- Herring G. M. A comparison of bone matrix and tendon with particular reference to glycoprotein content. Biochem J. 1976 Dec 1;159(3):749–755. doi: 10.1042/bj1590749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herring G. M. Methods for the study of the glycoproteins and proteoglycans of bone using bacterial collagenase. Determination of bone sialoprotein and chondroitin sulphate. Calcif Tissue Res. 1977 Dec 14;24(1):29–36. doi: 10.1007/BF02223293. [DOI] [PubMed] [Google Scholar]
- Huggins C. B., Urist M. R. Dentin matrix transformation: rapid induction of alkaline phosphatase and cartilage. Science. 1970 Feb 6;167(3919):896–898. doi: 10.1126/science.167.3919.896. [DOI] [PubMed] [Google Scholar]
- Lejeune E., Anjou A., Bouvier M., Robert J., Vauzelle J. L., Jeanneret J., Poizat J. P. Une nouvelle observation de dysplasie cranio-métaphysaire familiale. J Radiol Electrol Med Nucl. 1968 Jun-Jul;49(6):493–498. [PubMed] [Google Scholar]
- Olsen B. R., Guzman N. A., Engel J., Condit C., Aase S. Purification and characterization of a peptide from the carboxy-terminal region of chick tendon procollagen type I. Biochemistry. 1977 Jun 28;16(13):3030–3036. doi: 10.1021/bi00632a034. [DOI] [PubMed] [Google Scholar]
- Owen M., Shetlar M. R. Uptake of 3H-glucosamine by osteoclasts. Nature. 1968 Dec 28;220(5174):1335–1336. doi: 10.1038/2201335a0. [DOI] [PubMed] [Google Scholar]
- Peterkofsky B., Diegelmann R. Use of a mixture of proteinase-free collagenases for the specific assay of radioactive collagen in the presence of other proteins. Biochemistry. 1971 Mar 16;10(6):988–994. doi: 10.1021/bi00782a009. [DOI] [PubMed] [Google Scholar]
- Reddi A. H., Huggins C. Biochemical sequences in the transformation of normal fibroblasts in adolescent rats. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1601–1605. doi: 10.1073/pnas.69.6.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanford C. The hydrophobic effect and the organization of living matter. Science. 1978 Jun 2;200(4345):1012–1018. doi: 10.1126/science.653353. [DOI] [PubMed] [Google Scholar]
- Triffitt J. T., Owen M. Studies on bone matrix glycoproteins. Incorporation of (1-14C)glucosamine and plasma (14C)glycoprotein into rabbit cortical bone. Biochem J. 1973 Sep;136(1):125–134. doi: 10.1042/bj1360125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urist M. R. Bone: formation by autoinduction. Science. 1965 Nov 12;150(3698):893–899. doi: 10.1126/science.150.3698.893. [DOI] [PubMed] [Google Scholar]
- Urist M. R., Dowell T. A., Hay P. H., Strates B. S. Inductive substrates for bone formation. Clin Orthop Relat Res. 1968 Jul-Aug;59:59–96. [PubMed] [Google Scholar]
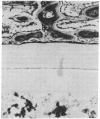
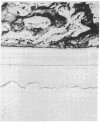
- Urist M. R., Granstein R., Nogami H., Svenson L., Murphy R. Transmembrane bone morphogenesis across multiple-walled diffusion chambers. New evidence for a diffusible bone morphogenetic property. Arch Surg. 1977 May;112(5):612–619. doi: 10.1001/archsurg.1977.01370050072012. [DOI] [PubMed] [Google Scholar]
- Urist M. R., Hay P. H., Dubuc F., Buring K. Osteogenetic competence. Clin Orthop Relat Res. 1969 May-Jun;64:194–220. [PubMed] [Google Scholar]
- Urist M. R., Iwata H., Boyd S. D., Ceccotti P. L. Observations implicating an extracellular enzymic mechanism of control of bone morphogenesis. J Histochem Cytochem. 1974 Feb;22(2):88–103. doi: 10.1177/22.2.88. [DOI] [PubMed] [Google Scholar]
- Urist M. R., Iwata H., Ceccotti P. L., Dorfman R. L., Boyd S. D., McDowell R. M., Chien C. Bone morphogenesis in implants of insoluble bone gelatin. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3511–3515. doi: 10.1073/pnas.70.12.3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urist M. R., Jurist J. M., Jr, Dubuc F. L., Strates B. S. Quantitation of new bone formation in intramuscular implants of bone matrix in rabbits. Clin Orthop Relat Res. 1970 Jan-Feb;68:279–293. [PubMed] [Google Scholar]
- Urist M. R., Mikulski A., Conteas C. N. Reversible extinction of the morphogen in bone matrix by reduction and oxidation of disulfide bonds. Calcif Tissue Res. 1975 Nov 24;19(1):73–83. doi: 10.1007/BF02563992. [DOI] [PubMed] [Google Scholar]
- Urist M. R., Nogami H. Morphogenetic substratum for differentiation of cartilage in tissue culture. Nature. 1970 Mar 14;225(5237):1051–1052. doi: 10.1038/2251051a0. [DOI] [PubMed] [Google Scholar]
- Urist M. R., Silverman B. F., Büring K., Dubuc F. L., Rosenberg J. M. The bone induction principle. Clin Orthop Relat Res. 1967 Jul-Aug;53:243–283. [PubMed] [Google Scholar]
- Urist M. R., Strates B. S. Bone morphogenetic protein. J Dent Res. 1971 Nov-Dec;50(6):1392–1406. doi: 10.1177/00220345710500060601. [DOI] [PubMed] [Google Scholar]
- Urist M. R., Terashima Y., Nakagawa M., Stamos C. Cartilage tissue differentiation from mesenchymal cells derived from mature muscle in tissue culture. In Vitro. 1978 Aug;14(8):697–706. doi: 10.1007/BF02616166. [DOI] [PubMed] [Google Scholar]
- Urist M. R. The substratum for bone morphogenesis. Symp Soc Dev Biol. 1970;29:125–163. [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]