Abstract
Since diagnosis at an early stage still remains a key issue for modern oncology and is crucial for successful cancer therapy, development of sensitive, specific, and non-invasive tumor markers, especially, in serum, is urgently needed. Midkine (MK), a plasma secreted protein, was initially identified in embryonal carcinoma cells at early stages of retinoic acid-induced differentiation. Multiple studies have reported that MK plays important roles in tumor progression, and is highly expressed in various malignant tumors. Because increased serum MK concentrations also have been reported in patients with various tumors, serum MK may have the potential to become a very useful tumor marker. Here, we review and discuss the possibility and usefulness of MK as a novel tumor marker.
Keywords: Midkine, tumor marker, cancer screening, prognostic prediction
1. Introduction
Midkine (MK), a heparin-binding growth factor, was originally reported to be the product of a retinoic acid-responsive gene during embryogenesis [1,2]. In 1988, Kadomatsu et al. first isolated an MK cDNA clone by differential hybridization and reported that MK was intensely expressed in early differentiation stages of embryonal carcinoma cells [1]. Because the RNA was originally detected only in midgestation mouse embryos and the kidney in adults, MK was initially called “midgestation embryo and kidney (MK) gene” [2]. During the two decades since the discovery of MK, substantial advances have been made in understanding the biological activity and molecular basis of MK. It is well-documented that MK is a multifunctional peptide which, together with pleiotrophin (PTN), forms a structurally distinct family of heparin-binding growth factors [3]. Interestingly, despite its high expression during embryogenesis, MK is not detectable in healthy adults and only re-appears in the body as a part of the pathogenesis of diseases [3]. Moreover, the most intriguing feature of MK is its massive expression in advanced tumors with surprisingly-high frequency. In this review, we describethe molecular genetic and biological significance of MK, especially focusing on malignant tumor, and introduce the possibility of MK as a novel tumor marker.
2. Midkine
2.1. Molecular Genetic Characterization
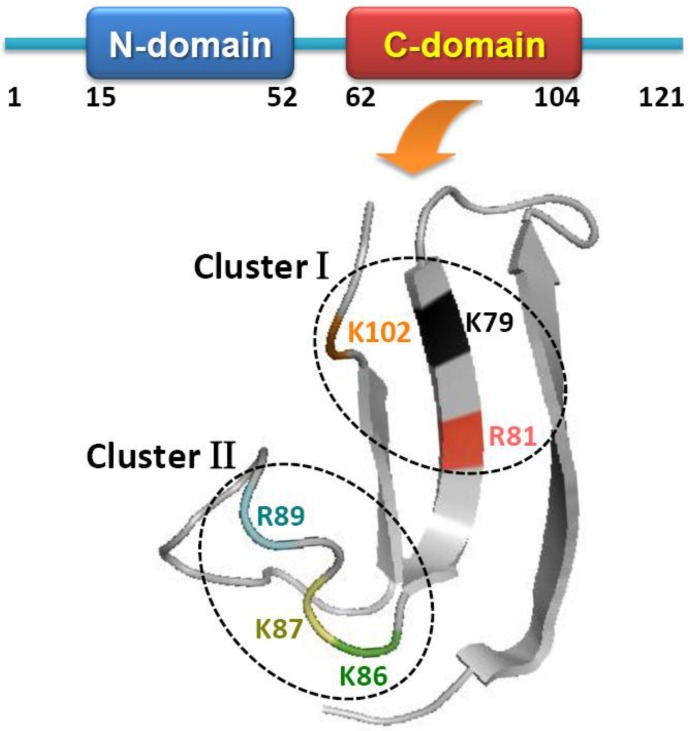
The human midkine gene is located on chromosome 11q11 and encodes a 13-kDa protein rich in a basic amino acid and cysteine [4,5,6]. MK is widely conserved from Drosophila to human [7]. Human and mouse MK sequences are extremely highly conserved: 87% of amino acids are identical and all amino acid changes are conservative except for an insertion [8]. MK is composed of two domains: an N-terminally located domain (MK 15–52), and a C-terminally-located domain (MK 62–104) flanked by intra-domain disulfide bridges [9] (Figure 1). The three dimensional structure of MK has been clarified based on the structures of the N-terminal half and C-terminal half molecules determined by NMR [10]. In the C-terminal half of human MK, two heparin-binding clusters, namely cluster I (K79, R81 and K102) and cluster II (K86, K87 and R89), have been identified [10,11]. Cluster I is especially essential for the recognition of heparin sulfate as well as chondroitin sulfate proteoglycans, and responsible for multiple biological functions, such as neurite outgrowth, fibrinolysis, and nerve cell migration [11,12,13].
Figure 1.
The domain structure of MK and the three-dimensional structure of its C-terminal domain.
It is also well-documented that the expression of MK is developmentally regulated [1,2]. MK is highly expressed in the midgestation period in response to retinoic acid, whereas its expression becomes low or undetectable in normal adult tissues [3]. In the promoter region of the MK gene, there is a functional retinoic acid-responsive element, which is responsible for the MK expression induced by retinoic acid [14,15]. The 5’ regulatory region of the MK promoter also contains two binding sites for Wilms' tumor suppressor gene (WT1) [16]. The WT1-responsive element near the transcription start site of the MK promoter is required for reduction of MK expression by WT1. In addition, the transcriptional activity of the MK promoter is regulated by cell growth in part through p53-dependent pathways [17]. Recent studies also revealed that MK expression is regulated by hypoxia, cortisol, growth factors, and cytokines via a nuclear factor-κB (NF-κB) dependent pathway [18,19,20]. The precise regulatory mechanism underlying the tight regulation of MK expression remains to be elucidated.
2.2. Mechanism of Action
MK, a secreted plasma protein, initiates signaling through the ligand-dependent activation of receptors to participate in regulating diverse biological processes [7]. Several MK-binding cell surface proteins, including syndecans, integrins, protein tyrosine phosphatase ζ (PTPζ), anaplastic lymphomakinase (ALK), and low-density lipoprotein (LDL)-receptor-related protein (LRP) [7,21], have beenidentified. MK strongly binds to syndecan proteins, namely syndecan-1, -3, and -4 [22,23,24]. The binding of MK to syndecans is mediated by the heparan sulfate chains. PTPζ is a receptor-type protein tyrosine phosphatase of which the extracellular domain carries chondroitin sulfate chains, and the intracellular domain exhibits protein tyrosine phosphatase activity [7]. The chondroitin sulfate chains on its ectodomain of PTPζ are essential for MK-binding [13]. The interaction between MK and PTPζ activates phosphoinositide 3-kinase (PI3-kinase) and extracellular signal-regulated kinase (ERK) for osteoblast cell migration and neural survival [25,26]. ALK, a transmembrane tyrosine kinase, was identified to be a receptor for MK and PTN [27,28]. By binding of MK to ALK, PI3-kinase and ERK are activated for intracellular signaling and regulate cell growth [28]. In addition, LRP has been identified as a membrane protein, which was purified from the MK-affinity column [29]. LRP is a member of the LDL receptor family that accomplishes endocytosis of a diverse array of ligands [30]. LRP1 mediates MK endocytosis, and endocytosed MK enters the nucleus where it exerts anti-apoptotic activity [31]. Chen et al. reported that MK exhibits strong affinity for the N-terminal half of the second domain among the four ligand-binding domains of LRP1, and plays an important role in anchorage-independent cell growth [32]. Taken together, these receptors described above may regulate the biological activities of MK either independently or cooperatively. Further investigations are needed to specify the functional interaction and complex formation between these receptors.
2.3. Biological Significance
During the midgestation period of embryogenesis, MK plays important roles in the development of tooth, lung, kidney, bone and nerve tissues [33,34,35,36,37,38]. Morphogenesis and cell differentiation were inhibited in tooth germs cultured in the presence of neutralizing antibodies for MK [33]. In development of embryonic mouse lung, MK showed a weak effect on branching morphogenesis, but exhibited an effect in restoring development of mesenchymal tissue [34]. MK was also involved in kidney development via the molecular cascade of the epithelial conversion of the metanephric blastema [36]. In addition to the specific functions of MK in development, extensive evidence has also accumulated that MK has a huge variety of biological function, such as transformation, neural survival, tissue remodeling, cell growth, differentiation, cell migration, and carcinogenesis [3,7,19,39,40]. Kaneda et al. reported that MK exhibits nerve cell adhesion and guidance activity for neurite outgrowth via the heparin-like domain on nerve cell surface heparan sulfate proteoglycan [41]. MK, which has neuroprotective activity and neurite extension, expresses strongly cerebral infarct and Alzheimer’s disease [25,42,43,44,45]. After ischemic injury, MK is up-regulated by astrocytes in the surviving region of the cerebral cortex and the hippocampal CA1 subregion, suggesting that MK may contribute to the survival of injured neurons [43,46,47]. In the inflammatory response, MK promotes the cell migration of inflammatory cells, specifically macrophages and neutrophils [48,49]. In addition, MK expression increases at intraperitoneal adhesions after surgery and cardiac remodeling after myocardial infarction [50,51,52]. Most importantly, MK is significantly up-regulated in various malignant tumors and plays crucial roles in carcinogenesis [53,54,55,56]. Thus, due to its multifunctional properties, MK is deeply involved in the pathogenesis of various diseases, especially malignant tumors.
In accordance with its high expression in various malignant tumors, MK exerts cancer-related activities in the process of carcinogenesis, including transformation, fibrinolysis, cell migration, cell survival, anti-apoptosis, and angiogenesis [7,25,29,39,57,58,59,60,61]. Kadomatsu et al. reported that NIH3T3 cells were transformed by overexpression of MK, and formed tumors in nude mice [39]. MK serves as a mediator of retinoid and cooperates with basic fibroblast growth factor (bFGF) to enhance fibrinolytic activity of endothelial cells [57]. MK enhances the plasminogen activator (PA)/plasmin levels in bovine endothelial cells in a dose- and time-dependent manner, and the fibrinolytic activity can be achieved through this up-regulation of urokinase-type PA expression [57]. In addition, cell migration-promoting activity of MK has been demonstrated in neutrophils, osteoblastic osteosarcoma cells, neural cells, macrophages, and smooth muscle cells [13,38,40,49,58,62]. In primary neuronal cultures, MK inhibited caspase-dependent apoptosis via the activation of ERK and PI3-kinase [25]. Qi et al. also reported that MK rescued Wilms' tumor cells from cisplatin-induced apoptosis by regulating Bcl-2 expression [63]. Furthermore, angiogenic role of midkine has been shown by the observation that MK transfection into the breast carcinoma line, MCF-7, accelerated tumor growth, and increased tumor vascularity after implantation of the cells in nude mice [64]. Taken together, these distinct observations not only bring new insights into the novel biological roles of MK, but also open up novel therapeutic targets for the various malignant tumors.
3. Midkine as a Novel Tumor Marker
Numerous studies have demonstrated that MK expression in various malignant tumors is significantly higher than that in normal tissues [53,54,55,56]. Overexpression of MK at both mRNA and protein levels was reported in a variety of human malignancies, such as oral, gastrointestinal, hepatobiliary, lung, thyroid, bladder, cervical, ovarian, and prostate cancer [53,55,56,65,66,67,68,69,70,71,72,73]. Moreover, MK protein expression has been shown to be strongly correlated with poor prognosis in patients with neuroblastomas, astrocytomas, pancreatic head carcinomas, or gastrointestinal stromal tumors [74,75,76,77]. It should be noted that normal tissues of human adults show restricted expression of MK, whereas most carcinoma specimens express MK at significantly high levels in a tissue type-independent manner [53,54,64,78]. In addition to the characteristics of MK expression, because MK protein is a plasma-secreted protein, MK concentrations in blood may increase in patients with malignant diseases. Enzyme-linked immunoassay (EIA) has allowed measurement of MK levels in blood, and increased blood MK concentrations were reported in patients with malignant tumors, including hepatocellular, gastric, and lung carcinoma [79,80]. A series of findings suggests that MK in blood may have potential to become a sensitive and useful tumor marker. Accordingly, Ikematsu et al. reported that higher plasma MK concentrations in patients with neuroblastoma were strongly correlated with poor survival [81]. Thus, it is suggested that blood MK concentrations may also become a novel diagnostic marker for predicting prognosis of patients with various cancers described in this section.
3.1. Neuroblastoma
Neuroblastoma is the most common extracranial solid tumor in children, comprising between 8% and 10% of all childhood cancers, and accounts for 15% of all childhood cancer deaths, indicating the poor prognosis of many of the tumors [82,83]. A number of genetic and biological features have been recently investigated in an effort to reveal the pathogenesis of neuroblastoma and to identify useful tumor markers. Many prognostic studies have identified several tumor markers associated with overall or disease-free survival, including MYCN copy number, tyrosine kinase A (TrkA) expression level, ploidy, and deletion or loss of heterozygosity of chromosome 1p and gain of chromosome 17q [82]. However, because invasive diagnostic procedures, such as a tumor biopsy, are required for these tumor markers [83,84], development of more useful and non-invasive tumor markers is urgently needed to improve the prognosis of patients with neuroblastoma.
Various studies have confirmed that MK is highly expressed in neuroblastoma [74,81,86]. Nakagawa et al. first reported that MK mRNA expression was elevated in neuroblastoma specimens at all stages [74]. In agreement with its high mRNA expression, the plasma MK concentrations become elevated with advancing neuroblastoma stages [81]. A higher level of MK was correlated with MYCN amplification, low expression of TrkA, diploidy/tetraploidy, and older age, which are known prognostic factors for neuroblastoma, indicating that the elevated plasma MK concentrations is correlated with poor prognostic factors of neuroblastomas. Furthermore, Ikematsu et al. also confirmed there was a striking correlation between high plasma MK level and poor prognosis [86]. Analysis for sporadic neuroblastoma cases also showed that the MK level was also remarkably higher than in non-tumor controls, and correlated with the statuses of MYCN amplification and stage. A significant correlation was also observed between high plasma MK level and poor prognosis of sporadic neuroblastoma. Taken together, these reports strongly support that plasma MK level is a prognostic factor for neuroblastoma.
3.2. Hepatocellular Carcinoma
Hepatocellular carcinoma (HCC) is a common primary cancer of the liver. HCC, endemic to Asia and Africa with a rising incidence in Western countries, is one of the most common and aggressive cancers worldwide [87,88]. The high mortality associated with HCC is ascribed to the difficulty to diagnose at an early stage. Indeed, although HCC patients diagnosed at an early stage and thus receiving curative resection exhibit a significantly improved life prognosis, most symptomatic HCC patients are diagnosed at an advanced stage, precluding their chance for surgical intervention [89,90]. Therefore, early diagnosis has been considered as the most important factor to achieve long-term survival for HCC patients.
Even though α-fetoprotein (AFP) and protein induced by vitamin K absence-2 (PIVKA-II) are one of serologic markers widely used for diagnosing HCC patients, the elevated serum AFP is only observed in about 60% to 70% of HCC patients and, to a lesser extent (33–65%), in patients with smaller HCCs [92]. Moreover, non-specific elevation of serum AFP has been found in 15% to 58% of patients with chronic hepatitis and 11% to 47% of patients with liver cirrhosis [92], indicating the necessity of developing a specific and sensitive HCC biomarkers. Aridome et al. first reported that the MK mRNA level was higher in HCC specimens than in the corresponding non-cancerous tissues [67]. By immunohistochemical analysis, high expression of MK was observed in specimens from HCC patients [68]. A highly sensitive EIA for MK revealed that 0.6–0.8 ng/mL of MK was detected in serum samples in the majority of HCC cases, whereas the MK levels in the sera of normal human subjects were low or undetectable [79]. In addition, the overexpression level of MK in HCC with intra-hepatic metastasis was significantly higher than that in HCC without intra-hepatic metastasis [92]. It is noteworthy that a significant increase in serum MK is associated with HCC patients, including those with normal serum AFP concentrations [93]. The measurement of serum MK concentrations allows for distinguishing normal and cirrhosis individuals from HCC patients, including those with normal AFP and small tumors from two independent cohorts. Therefore, the combined score of multiple markers, such as MK and AFP and/or PIVKA-II, may improve the prediction accuracy of identifying HCC patients.
3.3. Esophageal Squamous Cell Carcinoma
Esophageal carcinoma is one of the most lethal malignant tumors in the gastrointestinal carcinoma family [94]. Once diagnosed with esophageal squamous cell carcinoma (ESCC), the prognosis is very poor, with a five-year survival rate below 10% [95,96]. Thus, identification of the novel sensitive markers with the use of a relatively non-invasive technique, are needed for detecting the presence of ESCC early. The MK mRNA level is higher in esophageal carcinoma specimens than in the corresponding non-cancerous tissues [67,78]. Likewise, esophageal tumor specimens were positively stained with anti-MK antibody by immunohistochemistry, while surrounding normal esophageal tissues in these specimens were not stained [97]. Additionally, Shimada et al. revealed that MK expression by the tumor was significantly associated with high level of serum MK and high serum MK concentrations were associated with tumor size, immunoreactivity, and poor survival [98]. It should be noted that the positive rate of serum MK concentrations was significantly higher than any of conventional markers, such as carcinoembryonic antigen (CEA), SCC antigen (SCC-Ag), and cytokeratin 19 fragment (CYFRA21-1). Multivariate analysis indicated that S-MK is an independent prognostic factor [98]. Since serum concentrations of some types of growth factors are useful to predict treatment response and patients’ outcome in ESCC, serum MK may be a useful prognostic marker for ESCC.
3.4. Oral Squamous Cell Carcinoma
Oral cancer is one of the common types of human cancer [99,100]. Because the survival rate for patients with oral cancer still remains poor, improved survival of patients with oral cancer requires better techniques for the prediction of prognosis. Oral squamous cell carcinoma (OSCC), the most common of several types of oral cancers, is associated with bad prognosis. Although previous studies have demonstrated the usefulness of tumor-associated antigens for primary diagnosis of OSCC, no tumor markers have given a precise prediction of prognosis [78,101]. Ota et al. provided the direct evidence that serum MK concentrations may have the potential to become a very useful tumor marker in OSCC [66]. Serum MK concentrations were significantly higher in patients with OSCC than in healthy controls. Patients in high serum MK groups showed a significantly lower five-year survival rate compared with patients in low serum MK groups. MK expression in blood and cancer tissues is indicative of a strong relationship with malignant potential, and high expression suggests a bad prognosis. Serum MK concentrations may thus be a useful marker not only for cancer screening but also for predicting prognosis of OSCC patients.
3.5. Breast Cancer
Breast cancer is a complex genetic disease characterized by the accumulation of multiple molecular alterations [102]. Although well-established clinicopathological factors show strong overall association with patients’ prognosis and outcome, it has become clear that patients with similar features may show distinct outcomes and vary in their response to therapy [103]. In order to improve the poor prognosis of breast cancer, molecular biomarkers including hormone receptors and human epidermal growth factor receptor 2, are assessed and used in routine clinical practice currently [104]. Although several additional biomarkers are extensively studied, only few biomarkers are useful. Ibusuki et al. demonstrated that measuring plasma MK levels in combination with conventional markers provided statistically significant improvement in the diagnosis of breast cancer [105]. Plasma MK levels were abnormally elevated in patients with breast cancer compared to healthy controls. Increased levels of MK were correlated with menopausal status and nuclear grade in primary invasive breast cancer without distant metastasis. In addition, cancer detection rates based on MK levels were higher than those based on three conventional markers including CEA, carbohydrate antigen 15-3 (CA15-3), and Nation Cancer Center-Stomach-439 (NCC-ST-439). Moreover, detection rates of breast cancer using a combination of two conventional tumor markers (CA15-3/CEA, CA15-3/NCC-ST-439, or CEA/NCC-ST-439) with MK was significantly higher than those using combination of three conventional tumor markers. Further clinical validation studies are needed to establish the clinical significance of MK in the plasma of patients with breast cancer.
3.6. Pancreatic Cancer
Pancreatic cancer is one of the most aggressive malignancies and has a five-year survival rate of 1–4% [106]. Although surgery is the only curative treatment for patients with pancreatic cancer, only approximately 10–20% of patients have surgically resectable disease at the time of initial presentation, and even in these cases, the five-year survival rate is only 20%. Therefore, development of novel diagnostic methods is urgently needed for the patients to undergo the surgery at the early stage of the disease. Ohhashi et al. investigated the feasibility of quantitative analysis of MK mRNA by quantitative real-time RT-PCR (qRT-PCR) as a promising tool for pancreatic cancer diagnosis [107]. Several pancreatic cancer cell lines originating from metastatic lesions, such as KP-3, AsPC-1, SUIT2, and CFPAC1, showed especially high levels of MK mRNA expression. The median value of MK mRNA expression in pancreatic cancer tissue was 5.4-fold higher than those of pancreatic non-neoplastic tissues. These data suggest that quantitative analysis of MK mRNA provides an objective and sensitive evaluation and may be a promising modality for the diagnosis of pancreatic cancer and the prediction of its prognosis. Further investigation on correlation between elevated plasma MK concentrations and prognosis of pancreatic cancer will strengthen the usefulness of MK as a novel prognostic marker.
4. Conclusions
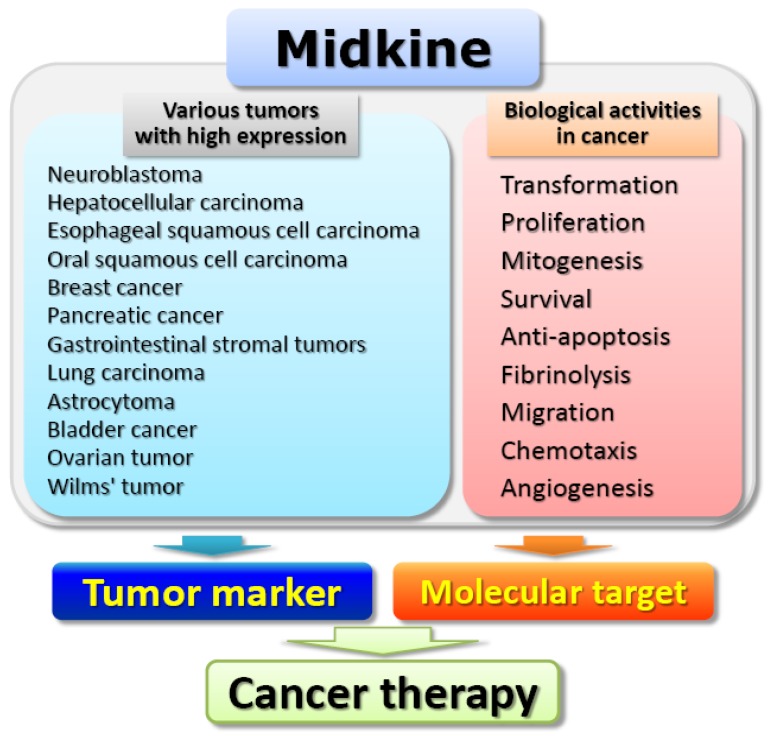
In this review, we described the unique biological features and clinical significance of MK, particularly focusing on the possibility of MK as a novel tumor marker. More additional studies have demonstrated that a truncated form of MK (t-MK), which lacks exon 3 encoding the N-terminus, is also found at both mRNA and protein levels in various tumors, including colon, breast, gastric, liver, and kidney, but not in normal specimens [108,109,110,111,112,113]. Because the frequency of the t-MK expression increases during tumor progression, t-MK may also be utilized as a tumor marker, especially to detect metastatic foci [111,114]. Furthermore, due to its biological significance in carcinogenesis, there is a growing body of evidence that MK can be regarded as a candidate molecular target for therapy against human carcinoma. Indeed, antisense MK oligodeoxyribonucleotides show anti-tumor activity for neurofibroma derived-cells and mouse rectal carcinoma cells [114,115]. Small interfering RNA (siRNA) mediated inhibition of MK expression also has an antitumor effect in prostate and gastric cancer [116,117]. In addition, MK promoter-based conditionally replicative adenovirus therapy for MK high expressing tumors has been attempted recently [118,119,120,121,122,123]. The 5’ regulatory region of the human MK promoter regulates and determines its tumor-specific MK expression. Because of the tumor-specific MK expression, the MK promoter-thymidine kinase gene exhibits a less side effect, indicating that a suicide gene delivery under the control of the MK promoter is a high potential strategy for cancer therapy. Collectively, a series of evidence described above strongly support the possibility of clinical application of MK (Figure 2). Understanding more biological feature and clinical significance of MK may not only bring new insights into the development of a sensitive and specific tumor marker, but may also open up novel therapeutic strategies for a large number of diseases.
Figure 2.
The clinical significance of MK in cancer therapy.
Acknowledgements
This work was supported by Grants-in-Aid for Scientific Research (B) 17390254 (Y. Ando) and (B) 17390541 (M. Shinohara) from the Ministry of Education, Science, Sports, and Culture of Japan. We are grateful to Kazutoshi Ota, Satoru Shinriki, and Tomoko Ota for their helpful input and discussions regarding the manuscript. Appreciation is extended to Cellmid Limited for providing midkine antibodies.
References
- 1.Kadomatsu K., Tomomura M., Muramatsu T. cDNA cloning and sequencing of a new gene intensely expressed in early differentiation stages of embryonal carcinoma cells and in mid-gestation period of mouse embryogenesis. Biochem. Biophys. Res. Commun. 1988;151:1312–1318. doi: 10.1016/S0006-291X(88)80505-9. [DOI] [PubMed] [Google Scholar]
- 2.Kadomatsu K., Huang R.P., Suganuma T., Murata F., Muramatsu T. A retinoic acid responsive gene MK found in the teratocarcinoma system is expressed in spatially and temporally controlled manner during mouse embryogenesis. J. Cell Biol. 1990;110:607–616. doi: 10.1083/jcb.110.3.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Muramatsu T. Midkine and pleiotrophin: two related proteins involved in development, survival, inflammation and tumorigenesis. J. Biochem. 2002;132:359–371. doi: 10.1093/oxfordjournals.jbchem.a003231. [DOI] [PubMed] [Google Scholar]
- 4.Matsubara S., Tomomura M., Kadomatsu K., Muramatsu T. Structure of a retinoic acid-responsive gene, MK, which is transiently activated during the differentiation of embryonal carcinoma cells and the mid-gestation period of mouse embryogenesis. J. Biol. Chem. 1990;265:9441–9443. [PubMed] [Google Scholar]
- 5.Kaname T., Kuwano A., Murano I., Uehara K., Muramatsu T., Kajii T. Midkine gene (MDK), a gene for prenatal differentiation and neuroregulation, maps to band 11p11. 2 by fluorescence in situ hybridization. Genomics. 1993;17:514–515. doi: 10.1006/geno.1993.1359. [DOI] [PubMed] [Google Scholar]
- 6.Murasugi A., Tohma-Aiba Y. Production of native recombinant human midkine in the yeast, Pichia pastoris. Protein Expr. Purif. 2003;27:244–252. doi: 10.1016/S1046-5928(02)00587-9. [DOI] [PubMed] [Google Scholar]
- 7.Kadomatsu K., Muramatsu T. Midkine and pleiotrophin in neural development and cancer. Cancer Lett. 2004;204:127–143. doi: 10.1016/S0304-3835(03)00450-6. [DOI] [PubMed] [Google Scholar]
- 8.Tsutsui J., Uehara K., Kadomatsu K., Matsubara S., Muramatsu T. A new family of heparin-binding factors: strong conservation of midkine (MK) sequences between the human and the mouse. Biochem. Biophys. Res. Commun. 1991;176:792–797. doi: 10.1016/S0006-291X(05)80255-4. [DOI] [PubMed] [Google Scholar]
- 9.Fabri L., Maruta H., Muramatsu H., Muramatsu T., Simpson R.J., Burgess A.W., Nice E.C. Structural characterization of native and recombinant forms of the neurotrophic cytokine MK. J. Chromatogr. 1993;646:213–226. doi: 10.1016/S0021-9673(99)87023-X. [DOI] [PubMed] [Google Scholar]
- 10.Iwasaki W., Nagata K., Hatanaka H., Inui T., Kimura T., Muramatsu T., Yoshida K., Tasumi M., Inagaki F. Solution structure of midkine, a new heparin-binding growth factor. EMBO J. 1997;16:6936–6946. doi: 10.1093/emboj/16.23.6936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Asai T., Watanabe K., Ichihara-Tanaka K., Kaneda N., Kojima S., Iguchi A., Inagaki F., Muramatsu T. Identification of heparin-binding sites in midkine and their role in neurite-promotion. Biochem. Biophys. Res. Commun. 1997;236:66–70. doi: 10.1006/bbrc.1997.6905. [DOI] [PubMed] [Google Scholar]
- 12.Akhter S., Ichihara-Tanaka K., Kojima S., Muramatsu H., Inui T., Kimura T., Kaneda N., Talukder A.H., Kadomatsu K., Inagaki F., Muramatsu T. Clusters of basic amino acids in midkine: roles in neurite-promoting activity and plasminogen activator-enhancing activity. J. Biochem. 1998;123:1127–1136. doi: 10.1093/oxfordjournals.jbchem.a022052. [DOI] [PubMed] [Google Scholar]
- 13.Maeda N., Ichihara-Tanaka K., Kimura T., Kadomatsu K., Muramatsu T., Noda M. A receptor-like protein-tyrosine phosphatase PTPzeta/RPTPbeta binds a heparin-binding growth factor midkine. Involvement of arginine 78 of midkine in the high affinity binding to PTPzeta. J. Biol. Chem. 1999;274:12474–12479. doi: 10.1074/jbc.274.18.12474. [DOI] [PubMed] [Google Scholar]
- 14.Matsubara S., Take M., Pedraza C., Muramatsu T. Mapping and characterization of a retinoic acid-responsive enhancer of midkine, a novel heparin binding growth/differentiation factor with neurotrophic activity. J. Biochem. 1994;115:1088–1096. doi: 10.1093/oxfordjournals.jbchem.a124462. [DOI] [PubMed] [Google Scholar]
- 15.Pedraza C., Matsubara S., Muramatsu T. A retinoic acid-responsive element in human midkine gene. J. Biochem. 1995;117:845–849. doi: 10.1093/oxfordjournals.jbchem.a124785. [DOI] [PubMed] [Google Scholar]
- 16.Adachi Y., Matsubara S., Pedraza C., Ozawa M., Tsutsui J., Takamatsu H., Noguchi H., Akiyama T., Muramatsu T. Midkine as a novel target gene for the Wilms’ tumor suppressor gene (WT1) Oncogene. 1996;13:2197–2203. [PubMed] [Google Scholar]
- 17.Yu L., Ugai S., O-Wang J., Namba M., Kadomatsu K., Muramatsu T., Matsubara S., Sakiyama S., Tagawa M. Cell growth- and P53-dependent transcriptional activity of the midkine promoter confers suicide gene expression in tumor cells. Oncol. Rep. 2003;10:1301–1305. [PubMed] [Google Scholar]
- 18.Kaplan F., Comber J., Sladek R., Hudson T.J., Muglia L.J., Macrae T., Gagnon S., Asada M., Brewer J.A., Sweezey N.B. The growth factor midkine is modulated by both glucocorticoid and retinoid in fetal lung development. Am. J. Respir. Cell Mol. Biol. 2003;28:33–41. doi: 10.1165/rcmb.2002-0047OC. [DOI] [PubMed] [Google Scholar]
- 19.Reynolds P.R., Mucenski M.L., Le Cras T.D., Nichols W.C., Whitsett J.A. Midkine is regulated by hypoxia and causes pulmonary vascular remodeling. J. Biol. Chem. 2004;279:37124–37132. doi: 10.1074/jbc.M405254200. [DOI] [PubMed] [Google Scholar]
- 20.You Z., Dong Y., Kong X., Beckett L.A., Gandour-Edwards R., Melamed J. Midkine is a NF-kappaB-inducible gene that supports prostate cancer cell survival. BMC Med. Genomics. 2008;1:6. doi: 10.1186/1755-8794-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muramatsu H., Zou P., Suzuki H., Oda Y., Chen G.Y., Sakaguchi N., Sakuma S., Maeda N., Noda M., Takada Y., Muramatsu T. Alpha 4 beta1- and alpha 6 beta1-integrins are functional receptors for midkine, a heparin-binding growth factor. J. Cell Sci. 2004;117:5405–5415. doi: 10.1242/jcs.01423. [DOI] [PubMed] [Google Scholar]
- 22.Mitsiadis T.A., Salmivirta M., Muramatsu T., Muramatsu H., Rauvala H., Lehtonen E., Jalkanen M., Thesleff I. Expression of the heparin-binding cytokines, midkine (MK) and HB-GAM (pleiotrophin) is associated with epithelial-mesenchymal interactions during fetal development and organogenesia. Development. 1995;121:37–51. doi: 10.1242/dev.121.1.37. [DOI] [PubMed] [Google Scholar]
- 23.Kojima T., Katsumi A., Yamazaki T., Muramatsu T., Nagasaka T., Ohsumi K., Saito H. Human ryudocan from endothelial-like cells binds basic fibroblast growth factor, midkine, and tissue factor pathway inhibitor. J. Biol. Chem. 1996;271:5914–5920. doi: 10.1074/jbc.271.10.5914. [DOI] [PubMed] [Google Scholar]
- 24.Nakanishi T., Kadomatsu K., Okamoto T., Ichihara-Tanaka K., Kojima T., Saito H., Tomoda Y., Muramatsu T. Expression of syndecan-1 and -3 during embryogenesis of the central nervous system in relation to binding with midkina. J. Biochem. 1997;121:197–205. [PubMed] [Google Scholar]
- 25.Owada K., Sanjo N., Kobayashi T., Mizusawa H., Muramatsu H., Muramatsu T., Michikawa M. Midkine inhibits caspase-dependent apoptosis via the activation of mitogenactivated protein kinase and phosphatidylinositol 3-kinase in cultured neurons. J. Neurochem. 1999;73:2084–2092. [PubMed] [Google Scholar]
- 26.Sakaguchi N., Muramatsu H., Ichihara-Tanaka K., Maeda N., Noda M., Yamamoto T., Michikawa M., Ikematsu S., Sakuma S., Muramatsu T. Receptor-type protein tyrosine phosphatase as a component of the signaling receptor complex for midkine-dependent survival of embryonicneurons. Neurosci. Res. 2003;45:219–224. doi: 10.1016/S0168-0102(02)00226-2. [DOI] [PubMed] [Google Scholar]
- 27.Stoica G.E., Kuo A., Aigner A., Sunitha I., Souttou B., Malerczyk C., Caughey D.J., Wen D., Karavanov A., Riegel A.T., et al. Identification of anaplastic lymphoma kinase as a receptor for the growth factor pleiotrophin. J. Biol. Chem. 2001;276:16772–16779. doi: 10.1074/jbc.M010660200. [DOI] [PubMed] [Google Scholar]
- 28.Stoica G.E., Kuo A., Powers C., Bowden E.T., Sale E.B., Riegel A.T., Wellstein A. Midkine binds to anaplastic lymphoma kinase (ALK) and acts as a growth factor for different cell types. J. Biol. Chem. 2002;277:35990–35998. doi: 10.1074/jbc.M205749200. [DOI] [PubMed] [Google Scholar]
- 29.Muramatsu H., Zou K., Sakaguchi N., Ikematsu S., Sakuma S., Muramatsu T. LDL receptor-related protein as a component of the midkine receptor. Biochem. Biophys. Res. Commun. 2000;270:936–941. doi: 10.1006/bbrc.2000.2549. [DOI] [PubMed] [Google Scholar]
- 30.Herz J., Beffert U. Apolipoprotein E receptors: linking brain development and Alzheimer's disease. Nat. Rev. Neurosci. 2000;1:51–58. doi: 10.1038/35036221. [DOI] [PubMed] [Google Scholar]
- 31.Shibata Y., Muramatsu T., Hirai M., Inui T., Kimura T., Saito H., McCormick L.M., Bu G., Kadomatsu K. Nuclear targeting by the growth factor midkine. Mol. Cell. Biol. 2002;22:6788–6796. doi: 10.1128/MCB.22.19.6788-6796.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen S., Bu G., Takei Y., Sakamoto K., Ikematsu S., Muramatsu T., Kadomatsu K. Midkine and LDL-receptor-related protein 1 contribute to the anchorage-independent cell growth of cancer cells. J. Cell Sci. 2007;120:4009–4015. doi: 10.1242/jcs.013946. [DOI] [PubMed] [Google Scholar]
- 33.Mitsiadis T.A., Muramatsu T., Muramatsu H., Thesleff I. Midkine (MK), a heparin-binding growth/differentiation factor, is regulated by retinoic acid and epithelial-mesenchymal interactions in the developing mouse tooth, and affects cell proliferation and morphogenesis. J. Cell Biol. 1995;129:267–281. doi: 10.1083/jcb.129.1.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Toriyama K., Muramatsu H., Hoshino T., Torii S., Muramatsu T. Evaluation of heparin-binding growth factors in rescuing morphogenesis of heparitinase-treated mouse embryonic lung explants. Differentiation. 1997;61:161–167. doi: 10.1046/j.1432-0436.1997.6130161.x. [DOI] [PubMed] [Google Scholar]
- 35.Sakurai H., Bush K.T., Nigam S.K. Identification of pleiotrophin as a mesenchymal factor involved in ureteric bud branching morphogenesis. Development. 2001;128:3283–3293. doi: 10.1242/dev.128.17.3283. [DOI] [PubMed] [Google Scholar]
- 36.Vilar J., Lalou C., Duong V.H., Charrin S., Hardouin S., Raulais D., Merlet-Bénichou C., Leliévre-Pégorier M. Midkine is involved in kidney development and in its regulation by retinoids. J. Am. Soc. Nephrol. 2002;13:668–676. doi: 10.1681/ASN.V133668. [DOI] [PubMed] [Google Scholar]
- 37.Dreyfus J., Brunet-de Carvalho N., Duprez D., Raulais D., Vigny M. HB-GAM/pleiotrophin but not RIHB/midkine enhances chondrogenesis in micromass culture. Exp. Cell Res. 1998;241:171–180. doi: 10.1006/excr.1998.4040. [DOI] [PubMed] [Google Scholar]
- 38.Imai S., Kaksonen M., Raulo E., Kinnunen T., Fages C., Meng X., Lakso M., Rauvala H. Osteoblast recruitment and bone formation enhanced by cell matrix-associated heparin-binding growth-associated molecule (HB-GAM) J. Cell Biol. 1998;143:1113–1128. doi: 10.1083/jcb.143.4.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kadomatsu K., Hagihara M., Akhter S., Fan Q.W., Muramatsu H., Muramatsu T. Midkine induces the transformation of NIH3T3 cells. Br. J. Cancer. 1997;75:354–359. doi: 10.1038/bjc.1997.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Qi M., Ikematsu S., Maeda N., Ichihara-Tanaka K., Sakuma S., Noda M., Muramatsu T., Kadomatsu K. Haptotactic migration induced by midkine. Involvement of protein-tyrosine phosphatase zeta. Mitogen-activated protein kinase, and phosphatidylinositol 3-kinase. J. Biol. Chem. 2001;276:15868–15875. doi: 10.1074/jbc.m005911200. [DOI] [PubMed] [Google Scholar]
- 41.Kaneda N., Talukder A.H., Nishiyama H., Koizumi S., Muramatsu T. Midkine, a heparin-binding growth/differentiation factor, exhibits nerve cell adhesion and guidance activity for neurite outgrowth in vitro. J. Biochem. 1996;119:1150–1156. doi: 10.1093/oxfordjournals.jbchem.a021361. [DOI] [PubMed] [Google Scholar]
- 42.Michikawa M., Kikuchi S., Muramatsu H., Muramatsu T., Kim S.U. Retinoic acid responsive gene product, midkine, has neurotrophic functions for mouse spinal cord and dorsal root ganglion neurons in culture. J. Neurosci. Res. 1993;35:530–539. doi: 10.1002/jnr.490350509. [DOI] [PubMed] [Google Scholar]
- 43.Yoshida Y., Goto M., Tsutsui J., Ozawa M., Sato E., Osame M., Muramatsu T. Midkine is present in the early stage of cerebral infarct. Brain Res. Dev. Brain Res. 1995;85:25–30. doi: 10.1016/0165-3806(94)00183-Z. [DOI] [PubMed] [Google Scholar]
- 44.Yasuhara O., Muramatsu H., Kim S.U., Muramatsu T., Maruta H., McGeer P.L. Midkine, a novel neurotrophic factor, is present in senile plaques of Alzheimer disease. Biochem. Biophys. Res. Commun. 1993;192:246–251. doi: 10.1006/bbrc.1993.1406. [DOI] [PubMed] [Google Scholar]
- 45.Salama R.H., Muramatsu H., Shimizu E., Hashimoto K., Ohgake S., Watanabe H., Komatsu N., Okamura N., Koike K., Shinoda N., et al. Increased midkine levels in sera from patients with Alzheimer's disease. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2005;29:611–616. doi: 10.1016/j.pnpbp.2005.01.018. [DOI] [PubMed] [Google Scholar]
- 46.Wang S., Yoshida Y., Goto M., Moritoyo T., Tsutsui J., Izumo S., Sato E., Muramatsu T., Osame M. Midkine exists in astrocytes in the early stage of cerebral infarction. Dev. Brain Res. 1998;106:205–209. doi: 10.1016/S0165-3806(97)00213-7. [DOI] [PubMed] [Google Scholar]
- 47.Mochizuki R., Takeda A., Sato N., Kimpara T., Onodera H., Itoyama Y., Muramatsu T. Induction of midkine expression in reactive astrocytes following rat transient forebrain ischemia. Exp. Neurol. 1998;149:73–78. doi: 10.1006/exnr.1997.6687. [DOI] [PubMed] [Google Scholar]
- 48.Takada T., Toriyama K., Muramatsu H., Song X.J., Torii S., Muramatsu T. Midkine, a retinoic acid-inducible heparin-binding cytokine in inflammatory responses: chemotactic activity to neutrophils and association with inflammatory synovitis. J. Biochem. 1997;122:453–458. doi: 10.1093/oxfordjournals.jbchem.a021773. [DOI] [PubMed] [Google Scholar]
- 49.Horiba M., Kadomatsu K., Nakamura E., Muramatsu H., Ikematsu S., Sakuma S., Hayashi K., Yuzawa Y., Matsuo S., Kuzuya M., et al. Neointima formation in a restenosis model is suppressed in midkine-deficient mice. J. Clin. Invest. 2000;105:489–495. doi: 10.1172/JCI7208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Inoh K., Muramatsu H., Ochiai K., Torii S., Muramatsu T. Midkine, a heparin-binding cytokine, plays key roles in intraperitoneal adhesions. Biochem. Biophys. Res. Commun. 2004;317:108–113. doi: 10.1016/j.bbrc.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 51.Obama H., Biro S., Tashiro T., Tsutsui J., Ozawa M., Yoshida H., Tanaka H., Muramatsu T. Myocardial infarction induces expression of midkine, a heparin-binding growth factor with reparative activity. Anticancer Res. 1998;18:145–152. [PubMed] [Google Scholar]
- 52.Horiba M., Kadomatsu K., Yasui K., Lee J.K., Takenaka H., Sumida A., Kamiya K., Chen S., Sakuma S., Muramatsu T., et al. Midkine plays a protective role against cardiac ischemia/reperfusion injury through a reduction of apoptotic reaction. Circulation. 2006;114:1713–1720. doi: 10.1161/CIRCULATIONAHA.106.632273. [DOI] [PubMed] [Google Scholar]
- 53.Garver R.I., Jr., Chan C.S., Milner P.G. Reciprocal expression of pleiotrophin and midkine in normal versus malignant lung tissues. Am. J. Respir. Cell Mol. Biol. 1993;9:463–466. doi: 10.1165/ajrcmb/9.5.463. [DOI] [PubMed] [Google Scholar]
- 54.Garver R.I., Jr., Radford D.M., Donis-Keller H., Wick M.R., Milner P.G. Midkine and pleiotrophin expression in normal and malignant breast tissue. Cancer. 1994;74:1584–1590. doi: 10.1002/1097-0142(19940901)74:5<1584::AID-CNCR2820740514>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 55.Konishi N., Nakamura M., Nakaoka S., Hiasa Y., Cho M., Uemura H., Hirao Y., Muramatsu T., Kadomatsu K. Immunohistochemical analysis of midkine expression in human prostate carcinoma. Oncology. 1999;57:253–257. doi: 10.1159/000012039. [DOI] [PubMed] [Google Scholar]
- 56.Ye C., Qi M., Fan Q.W., Ito K., Akiyama S., Kasai Y., Matsuyama M., Muramatsu T., Kadomatsu K. Expression of midkine in the early stage of carcinogenesis in human colorectal cancer. Br. J. Cancer. 1999;79:179–184. doi: 10.1038/sj.bjc.6690030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kojima S., Muramatsu H., Amanuma H., Muramatsu T. Midkine enhances fibrinolytic activity of bovine endothelial cells. J. Biol. Chem. 1995;270:9590–9596. doi: 10.1074/jbc.270.16.9590. [DOI] [PubMed] [Google Scholar]
- 58.Ohta S., Muramatsu H., Senda T., Zou K., Iwata H., Muramatsu T. Midkine is expressed during repair of bone fracture and promotes chondrogenesis. J. Bone Miner. Res. 1999;14:1132–1144. doi: 10.1359/jbmr.1999.14.7.1132. [DOI] [PubMed] [Google Scholar]
- 59.Ratovitski E.A., Kotzbauer P.T., Milbrandt J., Lowenstein C.J., Burrow C.R. Midkine induces tumor cell proliferation and binds to a high affinity signaling receptor associated with JAK tyrosine kinases. J. Biol. Chem. 1998;273:3654–3660. doi: 10.1074/jbc.273.6.3654. [DOI] [PubMed] [Google Scholar]
- 60.Huang Y., Hoque M.O., Wu F., Trink B., Sidransky D., Ratovitski E.A. Midkine induces epithelial-mesenchymal transition through Notch2/Jak2-Stat3 signaling in human keratinocytes. Cell Cycle. 2008;7:1613–1622. doi: 10.4161/cc.7.11.5952. [DOI] [PubMed] [Google Scholar]
- 61.Huang Y., Sook-Kim M., Ratovitski E. Midkine promotes tetraspanin-integrin interaction and induces FAK-Stat1alpha pathway contributing to migration/invasiveness of human head and neck squamous cell carcinoma cells. Biochem. Biophys. Res. Commun. 2008;377:474–478. doi: 10.1016/j.bbrc.2008.09.138. [DOI] [PubMed] [Google Scholar]
- 62.Sato W., Kadomatsu K., Yuzawa Y., Muramatsu H., Hotta N., Matsuo S., Muramatsu T. Midkine is involved in neutrophil infiltration into the tubulointerstitium in ischemic renal injury. J. Immunol. 2001;167:3463–3469. doi: 10.4049/jimmunol.167.6.3463. [DOI] [PubMed] [Google Scholar]
- 63.Qi M., Ikematsu S., Ichihara-Tanaka K., Sakuma S., Muramatsu T., Kadomatsu K. Midkine rescues Wilms' tumor cells from cisplatin-induced apoptosis: regulation of Bcl-2 expression by midkine. J. Biochem. 2000;127:269–277. doi: 10.1093/oxfordjournals.jbchem.a022604. [DOI] [PubMed] [Google Scholar]
- 64.Choudhuri R., Zhang H.T., Donnini S., Ziche M., Bicknell R. An angiogenic role for the neurokines midkine and pleiotrophin in tumorigenesis. Cancer Res. 1997;57:1814–1819. [PubMed] [Google Scholar]
- 65.Ruan M., Ji T., Wu Z., Zhou J., Zhang C. Evaluation of expression of midkine in oral squamous cell carcinoma and its correlation with tumour angiogenesis. Int. J. Oral Maxillofac Surg. 2007;36:159–164. doi: 10.1016/j.ijom.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 66.Ota K., Fujimori H., Ueda M., Shiniriki S., Kudo M., Jono H., Fukuyoshi Y., Yamamoto Y., Sugiuchi H., Iwase H., Shinohara M., et al. Midkine as a prognostic biomarker in oral squamous cell carcinoma. Br. J. Cancer. 2008;99:655–662. doi: 10.1038/sj.bjc.6604539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Aridome K., Tsutsui J., Takao S., Kadomatsu K., Ozawa M., Aikou T., Muramatsu T. Increased midkine gene expression in human gastrointestinal cancers. Jpn. J. Cancer Res. 1995;86:655–661. doi: 10.1111/j.1349-7006.1995.tb02449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kato M., Shinozawa T., Kato S., Awaya A., Terada T. Increased midkine expression in hepatocellular carcinoma. Arch. Pathol. Lab. Med. 2000;124:848–852. doi: 10.5858/2000-124-0848-IMEIHC. [DOI] [PubMed] [Google Scholar]
- 69.Kato M., Shinozawa T., Kato S., Endo K., Terada T. Increased midkine expression in intrahepatic cholangiocarcinoma: immunohistochemical and in situ hybridization analyses. Liver. 2000;20:216–221. doi: 10.1034/j.1600-0676.2000.020003216.x. [DOI] [PubMed] [Google Scholar]
- 70.Kato M., Maeta H., Kato S., Shinozawa T., Terada T. Immunohistochemical and in situ hybridization analyses of midkine expression in thyroid papillary carcinoma. Mod. Pathol. 2000;13:1060–1065. doi: 10.1038/modpathol.3880195. [DOI] [PubMed] [Google Scholar]
- 71.O'Brien T., Cranston D., Fuggle S., Bicknell R., Harris A.L. The angiogenic factor midkine is expressed in bladder cancer, and overexpression correlates with a poor outcome in patients with invasive cancers. Cancer Res. 1996;56:2515–2518. [PubMed] [Google Scholar]
- 72.Moon H.S., Park W.I., Sung S.H., Choi E.A., Chung H.W., Woo B.H. Immunohistochemical and quantitative competitive PCR analyses of midkine and pleiotrophin expression in cervical cancer. Gynecol. Oncol. 2003;88:289–297. doi: 10.1016/S0090-8258(02)00070-7. [DOI] [PubMed] [Google Scholar]
- 73.Nakanishi T., Kadomatsu K., Okamoto T., Tomoda Y., Muramatsu T. Expression of midkine and pleiotropin in ovarian tumors. Obstet. Gynecol. 1997;90:285–290. doi: 10.1016/S0029-7844(97)00237-8. [DOI] [PubMed] [Google Scholar]
- 74.Nakagawara A., Milbrandt J., Muramatsu T., Deuel T.F., Zhao H., Cnaan A., Brodeur G.M. Differential expression of pleiotrophin and midkine in advanced neuroblastomas. Cancer Res. 1995;55:1792–1797. [PubMed] [Google Scholar]
- 75.Mishima K., Asai A., Kadomatsu K., Ino Y., Nomura K., Narita Y., Muramatsu T., Kirino T. Increased expression of midkine during the progression of human astrocytomas. Neurosci. Lett. 1997;233:29–32. doi: 10.1016/S0304-3940(97)00619-8. [DOI] [PubMed] [Google Scholar]
- 76.Maeda S., Shinchi H., Kurahara H., Mataki Y., Noma H., Maemura K., Aridome K., Yokomine T., Natsugoe S., Aikou T., et al. Clinical significance of midkine expression in pancreatic head carcinoma. Br. J. Cancer. 2007;97:405–411. doi: 10.1038/sj.bjc.6603879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kaifi J.T., Fiegel H.C., Rafnsdottir S.L., Aridome K., Schurr P.G., Reichelt U., Wachowiak R., Kleinhans H., Yekebas E.F., Mann O., et al. Midkine as a prognostic marker for gastrointestinal stromal tumors. J. Cancer Res. Clin. Oncol. 2007;133:431–435. doi: 10.1007/s00432-006-0180-y. [DOI] [PubMed] [Google Scholar]
- 78.Tsutsui J., Kadomatsu K., Matsubara S., Nakagawara A., Hamanoue M., Takao S., Shimazu H., Ohi Y., Muramatsu T. A new family of heparin-binding growth/differentiation factors: increased midkine expression in Wilms' tumor and other human carcinomas. Cancer Res. 1993;53:1281–1285. [PubMed] [Google Scholar]
- 79.Muramatsu H., Song X.J., Koide N., Hada H., Tsuji T., Kadomatsu K., Inui T., Kimura T., Sakakibara S., Muramatsu T. Enzyme-linked immunoassay for midkine, and its application to evaluation of midkine levels in developing mouse brain and sera from patients with hepatocellular carcinomas. J. Biochem. 1996;119:1171–1175. doi: 10.1093/oxfordjournals.jbchem.a021364. [DOI] [PubMed] [Google Scholar]
- 80.Ikematsu S., Yano A., Aridome K., Kikuchi M., Kumai H., Nagano H., Okamoto K., Oda M., Sakuma S., Aikou T., et al. Serum midkine levels are increased in patients with various types of carcinomas. Br. J. Cancer. 2000;83:701–706. doi: 10.1054/bjoc.2000.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ikematsu S., Nakagawara A., Nakamura Y., Sakuma S., Wakai K., Muramatsu T., Kadomatsu K. Correlation of elevated level of blood midkine with poor prognostic factors of human neuroblastomas. Br. J. Cancer. 2003;88:1522–1526. doi: 10.1038/sj.bjc.6600938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Riley R.D., Heney D., Jones D.R., Sutton A.J., Lambert P.C., Abrams K.R., Young B., Wailoo A.J., Burchill S.A. A systematic review of molecular and biological tumor markers in neuroblastoma. Clin. Cancer Res. 2004;10:4–12. doi: 10.1158/1078-0432.CCR-1051-2. [DOI] [PubMed] [Google Scholar]
- 83.Grovas A., Fremgen A., Rauck A., Ruymann F.B., Hutchinson C.L., Winchester D.P., Menck H.R. The National Cancer Data Base Report on patterns of childhood cancer in the United States. Cancer. 1997;80:2321–2332. doi: 10.1002/(SICI)1097-0142(19971215)80:12<2321::AID-CNCR14>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 84.Brodeur G.M. Neuroblastoma: biological insights into a clinical enigma. Nat. Rev. Cancer. 2003;3:203–216. doi: 10.1038/nrc1014. [DOI] [PubMed] [Google Scholar]
- 85.Henry M.C., Tashjian D.B., Breuer C.K. Neuroblastoma update. Curr. Opin. Oncol. 2005;17:19–23. doi: 10.1097/01.cco.0000147901.12325.90. [DOI] [PubMed] [Google Scholar]
- 86.Ikematsu S., Nakagawara A., Nakamura Y., Ohira M., Shinjo M., Kishida S., Kadomatsu K. Plasma midkine level is a prognostic factor for human neuroblastoma. Cancer Sci. 2008;99:2070–2074. doi: 10.1111/j.1349-7006.2008.00957.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.El-Serag H.B., Mason A.C. Rising incidence of hepatocellular carcinoma in the United States. N. Engl. J. Med. 1999;340:745–750. doi: 10.1056/NEJM199903113401001. [DOI] [PubMed] [Google Scholar]
- 88.Parkin D.M., Bray F., Ferlay J., Pisani P. Global cancer statistics, 2002. CA Cancer J. Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 89.Poon R.T., Fan S.T., Lo C.M., Liu C.L., Wong J. Long-term survival and pattern of recurrence after resection of small hepatocellular carcinoma in patients with preserved liver function: implications for a strategy of salvage transplantation. Ann. Surg. 2002;235:373–382. doi: 10.1097/00000658-200203000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yuen M.F., Cheng C.C., Lauder I.J., Lam S.K., Ooi C.G., Lai C.L. Early detection of hepatocellular carcinoma increases the chance of treatment: Hong Kong experience. Hepatology. 2000;31:330–335. doi: 10.1002/hep.510310211. [DOI] [PubMed] [Google Scholar]
- 91.Taketa K. α-Fetoprotein: reevaluation in hepatology. Hepatology. 1990;12:1420–1432. doi: 10.1002/hep.1840120625. [DOI] [PubMed] [Google Scholar]
- 92.Yin Z., Luo X., Kang X., Wu Z., Qian H., Wu M. Correlation between midkine protein overexpression and intrahepatic metastasis in hepatocellular carcinoma. Zhonghua Zhong Liu Za Zhi. 2002;24:27–29. [PubMed] [Google Scholar]
- 93.Jia H.L., Ye Q.H., Qin L.X., Budhu A., Forgues M., Chen Y., Liu Y.K., Sun H.C., Wang L., Lu H.Z., et al. Gene expression profiling reveals potential biomarkers of human hepatocellular carcinoma. Clin. Cancer Res. 2007;13:1133–1139. doi: 10.1158/1078-0432.CCR-06-1025. [DOI] [PubMed] [Google Scholar]
- 94.Daly J.M., Karnell L.H., Menck H.R. National cancer data base report on esophageal carcinoma. Cancer. 1996;78:1820–1828. doi: 10.1002/(SICI)1097-0142(19961015)78:8<1820::AID-CNCR25>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 95.Hofstetter W., Swisher S.G., Correa A.M., Hess K., Putnam J.B., Jr., Ajani J.A., Dolormente M., Francisco R., Komaki R.R., Lara A., et al. Treatment outcomes of resected esophageal cancer. Ann. Surg. 2002;236:376–384. doi: 10.1097/00000658-200209000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lee J.M., Wu M.T., Lee Y.C., Yang S.Y., Chen J.S., Hsu H.H., Huang P.M., Kuo S.W., Lee C.J., Chen C.J. Association of GSTP1 polymorphism and survival for esophageal cancer. Clin. Cancer Res. 2005;11:4749–4753. doi: 10.1158/1078-0432.CCR-04-2333. [DOI] [PubMed] [Google Scholar]
- 97.Miyauchi M., Shimada H., Kadomatsu K., Muramatsu T., Matsubara S., Ikematsu S., Takenaga K., Asano T., Ochiai T., Sakiyama S., et al. Fequent expression of midkine gene in esophageal cancer suggests a potential usage of its promoter for suicide gene therapy. Jpn. J. Cancer Res. 1999;90:469–475. doi: 10.1111/j.1349-7006.1999.tb00771.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Shimada H., Nabeya Y., Tagawa M., Okazumi S., Matsubara H., Kadomatsu K., Muramatsu T., Ikematsu S., Sakuma S., Ochiai T. Preoperative serum midkine concentration is a prognostic marker for esophageal squamous cell carcinoma. Cancer Sci. 2003;94:628–632. doi: 10.1111/j.1349-7006.2003.tb01494.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Petersen P.E. The World Oral Health Report 2003: continuous improvement of oral health in the 21st century – the approach of the WHO Global Oral Health Programme. Community Dent. Oral. Epidemiol. 2003;31:3–23. doi: 10.1046/j..2003.com122.x. [DOI] [PubMed] [Google Scholar]
- 100.Jemal A., Siegel R., Ward E., Murray T., Xu J., Smigal C., Thun M.J. Cancer statistics. CA Cancer J. Clin. 2006;56:106–130. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- 101.Krimmel M., Hoffmann J., Krimmel C., Cornelius C.P., Schwenzer N. (1998) Relevance of SCC-Ag, CEA, CA 19.9 and CA 125 for diagnosis and follow-up in oral cancer. J. Craniomaxillofac. Surg. 1998;26:243–248. doi: 10.1016/S1010-5182(98)80020-6. [DOI] [PubMed] [Google Scholar]
- 102.Simpson P.T., Reis-Filho J.S., Gale T., Lakhani S.R. Molecular evolution of breast cancer. J. Pathol. 2005;205:248–254. doi: 10.1002/path.1691. [DOI] [PubMed] [Google Scholar]
- 103.Alizadeh A.A., Ross D.T., Perou C.M., van de Rijn M. Towards a novel classification of human malignancies based on gene expression patterns. J. Pathol. 2001;195:41–52. doi: 10.1002/path.889. [DOI] [PubMed] [Google Scholar]
- 104.Rakha E.A., Reis-Filho J.S., Ellis I.O. Combinatorial biomarker expression in breast cancer. Breast Cancer Res. Treat. 2010 doi: 10.1007/s10549-010-0746-x. in press. [DOI] [PubMed] [Google Scholar]
- 105.Ibusuki M., Fujimori H., Yamamoto Y., Ota K., Ueda M., Shinriki S., Taketomi M., Sakuma S., Shinohara M., Iwase H., et al. Midkine in plasma as a novel breast cancer marker. Cancer Sci. 2009;100:1735–1739. doi: 10.1111/j.1349-7006.2009.01233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Li D., Xie K., Wolff R., Abbruzzese J.L. Pancreatic cancer. Lancet. 2004;363:1049–1057. doi: 10.1016/S0140-6736(04)15841-8. [DOI] [PubMed] [Google Scholar]
- 107.Ohhashi S., Ohuchida K,, Mizumoto K., Egami T., Yu J., Cui L., Toma H., Takahata S., Nabae T., Tanaka M. Midkine mRNA is overexpressed in pancreatic cancer. Dig. Dis. Sci. 2009;54:811–815. doi: 10.1007/s10620-008-0434-4. [DOI] [PubMed] [Google Scholar]
- 108.Miyashiro I., Kaname T., Nakayama T., Nakamori S., Yagyu T., Monden T., Kikkawa N., Nishisho I., Muramatsu T., Monden M., et al. Expression of truncated midkine in human colorectal cancers. Cancer Lett. 1996;106:287–291. doi: 10.1016/0304-3835(96)04333-9. [DOI] [PubMed] [Google Scholar]
- 109.Kaname T., Kadomatsu K., Aridome K., Yamashita S., Sakamoto K., Ogawa M., Muramatsu T., Yamamura K. The expression of truncated MK in human tumors. Biochem. Biophys. Res. Commun. 1996;219:256–260. doi: 10.1006/bbrc.1996.0214. [DOI] [PubMed] [Google Scholar]
- 110.Miyashiro I., Kaname T., Shin E., Wakasugi E., Monden T., Takatsuka Y., Kikkawa N., Muramatsu T., Monden M., Akiyama T. Midkine expression in human breast cancers: expression of truncated form. Breast Cancer Res. Treat. 1997;43:1–6. doi: 10.1023/A:1005748728351. [DOI] [PubMed] [Google Scholar]
- 111.Aridome K., Takao S., Kaname T., Kadomatsu K., Natsugoe S., Kijima F., Aikou T., Muramatsu T. Truncated midkine as a marker of diagnosis and detection of nodal metastases in gastrointestinal carcinomas. Br. J. Cancer. 1998;78:472–477. doi: 10.1038/bjc.1998.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Paul S., Mitsumoto T., Asano Y., Kato S., Kato M., Shinozawa T. Detection of truncated midkine in Wilms' tumor by a monoclonal antibody against human recombinant truncated midkine. Cancer Lett. 2001;163:245–251. doi: 10.1016/S0304-3835(00)00696-0. [DOI] [PubMed] [Google Scholar]
- 113.Tao P., Xu D., Lin S., Ouyang G.L., Chang Y., Chen Q., Yuan Y., Zhuo X., Luo Q., Li J., et al. Abnormal expression, highly efficient detection and novel truncations of midkine in human tumors, cancers and cell lines. Cancer Lett. 2007;253:60–67. doi: 10.1016/j.canlet.2007.01.019. [DOI] [PubMed] [Google Scholar]
- 114.Mashour G.A., Ratner N., Khan G.A., Wang H.L., Martuza R.L., Kurtz A. (2001) The angiogenic factor midkine is aberrantly expressed in NFl-deficient Schwann cells and is a mitogen for neuronbroma-derived cells. Oncogene. 2001;20:97–105. doi: 10.1038/sj.onc.1204026. [DOI] [PubMed] [Google Scholar]
- 115.Takei Y., Kadomatsu K., Matsuo S., Itoh H., Nakazawa K., Kubota S., Muramatsu T. Antisense oligodeoxynucleotide targeted to Midkine, a heparin-binding growth factor, suppresses tumorigenicity of mouse rectal carcinoma cells. Cancer Res. 2001;61:8486–8491. [PubMed] [Google Scholar]
- 116.Takei Y., Kadomatsum K., Goto T., Muramatsu T. Combinational antitumor effect of siRNA against midkine and paclitaxel on growth of human prostate cancer xenografts. Cancer. 2006;107:864–873. doi: 10.1002/cncr.22068. [DOI] [PubMed] [Google Scholar]
- 117.Wang Q., Huang Y., Ni Y., Wang H., Hou Y. siRNA targeting midkine inhibits gastric cancer cells growth and induces apoptosis involved caspase-3, -8, -9 activation and mitochondrial depolarization. J. Biomed. Sci. 2007;14:783–795. doi: 10.1007/s11373-007-9192-0. [DOI] [PubMed] [Google Scholar]
- 118.Toyoda E., Doi R., Kami K., Mori T., Ito D., Koizumi M., Kida A., Nagai K., Ito T., Masui T., et al. Midkine promoter-based conditionally replicative adenovirus therapy for midkine-expressing human pancreatic cancer. J. Exp. Clin. Cancer Res. 2008;21:27–30. doi: 10.1186/1756-9966-27-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Adachi Y., Reynolds P.N., Yamamoto M., Grizzle W.E., Overturf K., Matsubara S., Muramatsu T., Curiel D.T. Midkine promoter-based adenoviral vector gene delivery for pediatric solid tumors. Cancer Res. 2000;60:4305–4310. [PubMed] [Google Scholar]
- 120.Adachi Y., Reynolds P.N., Yamamoto M., Wang M., Takayama K., Matsubara S., Muramatsu T., Curiel D.T. A midkine promoter-based conditionally replicative adenovirus for treatment of pediatric solid tumors and bone marrow tumor purging. Cancer Res. 2001;61:7882–7888. [PubMed] [Google Scholar]
- 121.Yu L., Hamada K., Namba M., Kadomatsu K., Muramatsu T., Matsubara S., Tagawa M. Midkine promoter-driven suicide gene expression and -mediated adenovirus replication produced cytotoxic effects to immortalised and tumour cells. Eur. J. Cancer. 2004;40:1787–1794. doi: 10.1016/j.ejca.2004.04.014. [DOI] [PubMed] [Google Scholar]
- 122.Kohno S., Nakagawa K., Hamada K., Harada H., Yamasaki K., Hashimoto K., Tagawa M., Nagato S., Furukawa K., Ohnishi T. Midkine promoter-based conditionally replicative adenovirus for malignant glioma therapy. Oncol. Rep. 2004;12:73–78. [PubMed] [Google Scholar]
- 123.Terao S., Shirakawa T., Kubo S., Bishunu A., Lee S.J., Goda K., Tsukuda M., Hamada K., Tagawa M., Takenaka A., et al. Midkine promoter-based conditionally replicative adenovirus for targeting midkine-expressing human bladder cancer model. Urology. 2007;70:1009–1013. doi: 10.1016/j.urology.2007.07.003. [DOI] [PubMed] [Google Scholar]