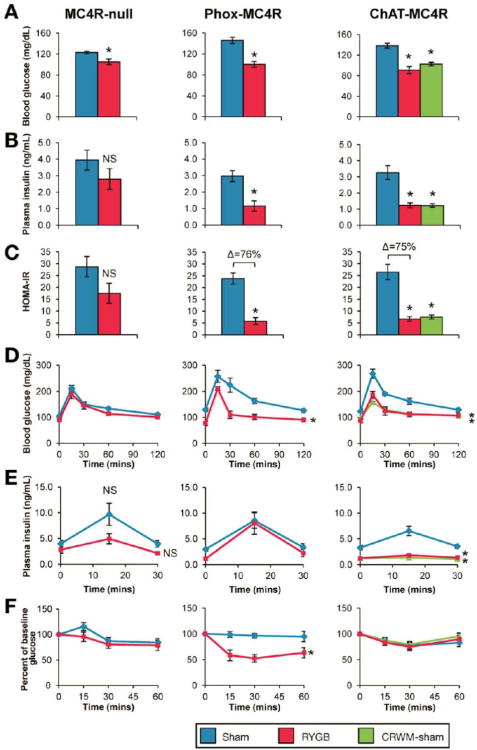
Figure 4. MC4Rs in parasympathetic vagal motor neurons mediate effects of RYGB on glucose homeostasis independent of changes in body weight.
(A-F, left panels) RYGB significantly reduced fasting glucose in MC4R-null mice, but failed to produce statistically-significant improvements in other measures of glucose homeostasis: insulin (B), HOMA-IR (C), glucose tolerance (D), glucose-stimulated plasma insulin (E), and insulin tolerance (F), presented as % of baseline glucose to control for differences in baseline glucose (n=6-14/group). (A-F, middle panels) In contrast, RYGB reduced fasting glucose (A) and insulin (B), and improved HOMA-IR (C), oral glucose tolerance (D), and insulin tolerance (F) in Phox-MC4R mice, despite a similar blunted weight-reduction as seen in MC4R-null mice. RYGB did not reduce their glucose-stimulated plasma insulin (n=6-7/group). (A-F, right panels) Consistent with their substantial weight reduction, fasting glucose (A), fasting plasma insulin (B), and glucose-stimulated plasma insulin (C) were reduced and oral glucose tolerance improved (D) in RYGB-treated ChAT-MC4R mice and CRWM-Sham ChAT-MC4R mice (n=6-7/group). *p<.05 vs. Sham.