Abstract
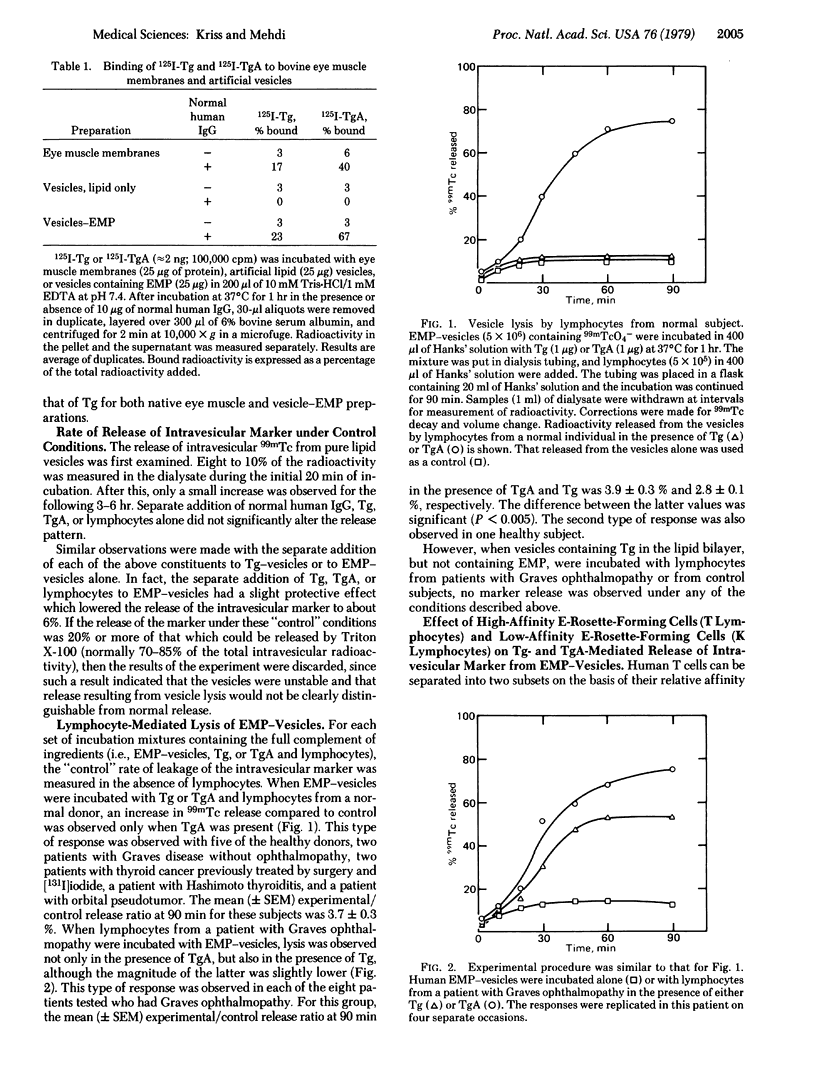
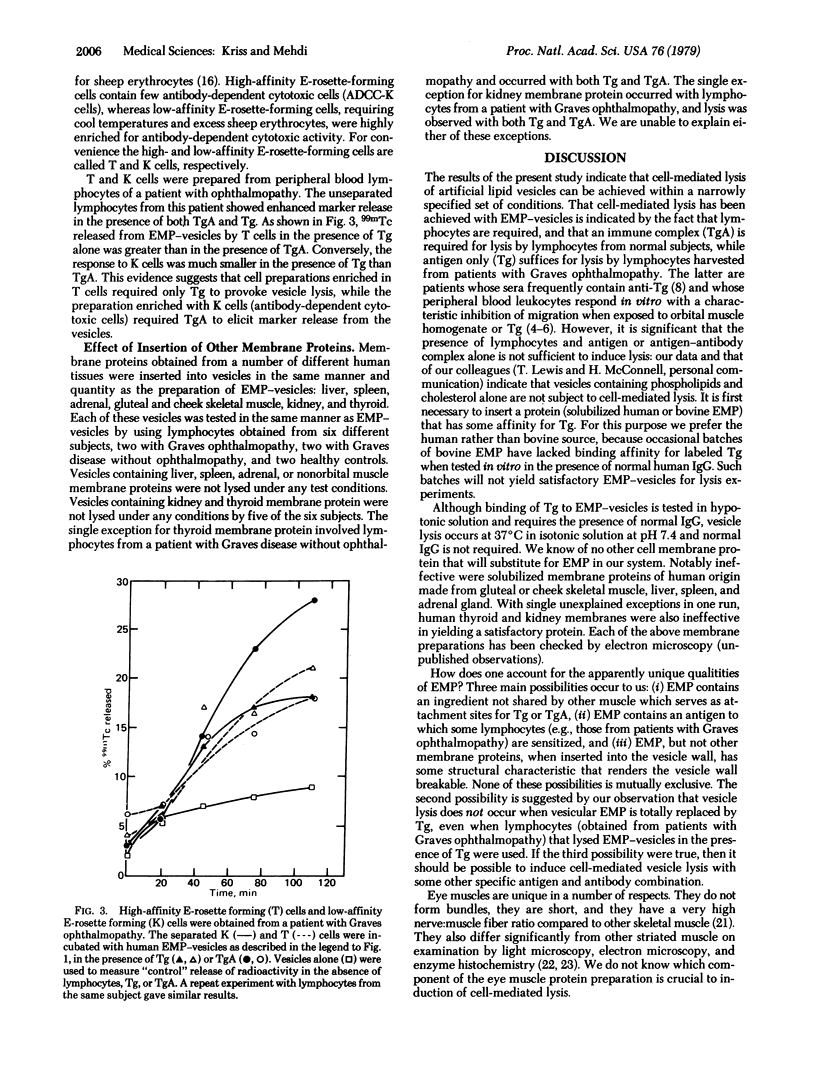
We prepared artificial vesicles that are lysed upon cell-mediated immunological attack by human lymphocytes. These vesicles are made from a mixture of dimyristoyl lecithin, dipalmitoyl lecithin, and cholesterol, have eye muscle membrane protein (EMP) inserted into the bilayer wall, and contain intravesicular 99mTc marker. Injury to the vesicular membrane was assessed by measurement of 99mTc release. Thyroglobulin (Tg) and Tg-anti-Tg complex (TgA) bind to EMP-vesicles to an extent equal to or greater than to native eye muscle membranes in vitro; this binding requires the presence of normal human IgG. The role of Tg, TgA, IgG, and peripheral blood lymphocytes in altering membrane permeability was analyzed. Incubation of vesicles for up to 3 hr alone, with added IgG alone, or with further addition of Tg or TgA did not result in 99mTc release. Addition of lymphocytes from normal donors to the above four preparations showed release in the presence of TgA. Lymphocytes from each of eight patients with Graves ophthalmopathy caused release not only in the presence of TgA, but also in the presence of Tg. Separation of a patient's lymphocytes into high- and low-affinity rosette-formers (T and K cells, respectively) showed that cell-mediated vesicle lysis in the presence of TgA was greater with K cells than with T cells, while vesicle lysis in the presence of Tg was greater with T cells than with K cells. Vesicles made with inserted Tg but lacking EMP were not lysed by such T cells. Lymphocytes failed to induce permeability changes in vesicles containing other inserted proteins obtained from human nonextraocular muscle, liver, spleen, or adrenal, even if Tg or TgA were present. The results support the concept that muscle cell damage in Graves ophthalmopathy is immunological, cell-mediated, and of two types: (i) K lymphocytes reacting to immune complex, TgA, on the eye muscle cell surface (i.e., antibody-dependent cytotoxicity) and (ii) sensitized T lymphocytes reacting to Tg on the eye muscle cell surface. An antigenic role for EMP is possible, but has not been unequivocally proven.
Keywords: T and K lymphocytes, thyroglobulin, antithyroglobulin, cell membranes, autoimmunity
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axén R., Porath J., Ernback S. Chemical coupling of peptides and proteins to polysaccharides by means of cyanogen halides. Nature. 1967 Jun 24;214(5095):1302–1304. doi: 10.1038/2141302a0. [DOI] [PubMed] [Google Scholar]
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Böyum A. Isolation and removal of lymphocytes from bone marrow of rats and guinea-pigs. Scand J Clin Lab Invest Suppl. 1968;97:91–106. [PubMed] [Google Scholar]
- Donaldson S. S., Bagshaw M. A., Kriss J. P. Supervoltage orbital radiotherapy for Graves' ophthalmopathy. J Clin Endocrinol Metab. 1973 Aug;37(2):276–285. doi: 10.1210/jcem-37-2-276. [DOI] [PubMed] [Google Scholar]
- Enzmann D., Donaldson S. S., Marshall W. H., Kriss J. P. Computed tomography in orbital pseudothumor (idiopathic orbital inflammation). Radiology. 1976 Sep;120(3):597–601. doi: 10.1148/120.3.597. [DOI] [PubMed] [Google Scholar]
- Enzmann D., Marshal W. H., Jr, Rosenthal A. R., Kriss J. P. Computed tomography in Graves' ophthalmopathy. Radiology. 1976 Mar;118(3):615–620. doi: 10.1148/118.3.615. [DOI] [PubMed] [Google Scholar]
- Hunter W. M., Greenwood F. C. A radio-immunoelectrophoretic assay for human growth hormone. Biochem J. 1964 Apr;91(1):43–56. doi: 10.1042/bj0910043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi J., Herman M. M., Kriss J. P. Binding of thyroglobulin and thyroglobulin-antithyroglobulin immune complex to extraocular muscle membrane. Endocrinology. 1974 Aug;95(2):434–446. doi: 10.1210/endo-95-2-434. [DOI] [PubMed] [Google Scholar]
- Kriss J. P., Konishi J., Herman M. Studies on the pathogenesis of Graves' ophthalmopathy (with some related observations regarding therapy). Recent Prog Horm Res. 1975;31:533–566. doi: 10.1016/b978-0-12-571131-9.50018-9. [DOI] [PubMed] [Google Scholar]
- Kriss J. P. Radioisotopic thyroidolymphography in patients with Graves' disease. J Clin Endocrinol Metab. 1970 Sep;31(3):315–323. doi: 10.1210/jcem-31-3-315. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Martinez A. J., Hay S., McNeer K. W. Extraocular muscles: light microscopy and ultrastructural features. Acta Neuropathol. 1976 Mar 30;34(3):237–253. doi: 10.1007/BF00688678. [DOI] [PubMed] [Google Scholar]
- Mashieu P., Winand R. Demonstration of delayed hypersensitivity to retrobulbar and thyroid tissues in human exophthalmos. J Clin Endocrinol Metab. 1972 Jun;34(6):1090–1092. doi: 10.1210/jcem-34-6-1090. [DOI] [PubMed] [Google Scholar]
- Mehdi S. Q., Nussey S. S. A radio-ligand receptor assay for the long-acting thyroid stimulator. Inhibition by the long-acting thyroid stimulator of the binding of radioiodinated thyroid-stimulating hormone to human thyroid membranes. Biochem J. 1975 Jan;145(1):105–111. doi: 10.1042/bj1450105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehdi S. Q., Nussey S. S., Shindelman J. E., Kriss J. P. The influence of lipid substitution on thyrotropin-receptor interactions in artificial vesicles. Endocrinology. 1977 Nov;101(5):1406–1412. doi: 10.1210/endo-101-5-1406. [DOI] [PubMed] [Google Scholar]
- Mori T., Kriss J. P. Measurements by competitive binding radioassay of serum anti-microsomal and anti-thyroglobulin antibodies in Graves' disease and other thyroid disorders. J Clin Endocrinol Metab. 1971 Oct;33(4):688–698. doi: 10.1210/jcem-33-4-688. [DOI] [PubMed] [Google Scholar]
- Mullin B. R., Levinson R. E., Friedman A., Henson D. E., Winand R. J., Kohn L. D. Delayed hypersensitivity in Graves' disease and exophthalmos: identification of thyroglobulin in normal human orbital muscle. Endocrinology. 1977 Feb;100(2):351–366. doi: 10.1210/endo-100-2-351. [DOI] [PubMed] [Google Scholar]
- Munro R. E., Lamki L., Row V. V., Volpé R. Cell-mediated immunity in the exophthalmos of Graves' disease as demonstrated by the migration inhibition factor (MIF) test. J Clin Endocrinol Metab. 1973 Aug;37(2):286–292. doi: 10.1210/jcem-37-2-286. [DOI] [PubMed] [Google Scholar]
- Pierce J. G., Rawitch A. B., Brown D. M., Stanley P. G. Human thyroglobulin: studies on the native and S-carboxymethylated protein. Biochim Biophys Acta. 1965 Nov 15;111(1):247–257. doi: 10.1016/0304-4165(65)90491-5. [DOI] [PubMed] [Google Scholar]
- Ringel S. P., Wilson W. B., Barden M. T., Kaiser K. K. Histochemistry of human extraocular muscle. Arch Ophthalmol. 1978 Jun;96(6):1067–1072. doi: 10.1001/archopht.1978.03910050587020. [DOI] [PubMed] [Google Scholar]
- Solomon D. H., Chopra I. J., Chopra U., Smith F. J. Identification of subgroups of euthyroid graves's ophthalmopathy. N Engl J Med. 1977 Jan 27;296(4):181–186. doi: 10.1056/NEJM197701272960401. [DOI] [PubMed] [Google Scholar]
- West W. H., Boozer R. B., Herberman R. B. Low affinity E-rosette formation by the human K cell. J Immunol. 1978 Jan;120(1):90–95. [PubMed] [Google Scholar]
- Winand R., Mahieu P. Prevention of malignant exophthalmos after treatment of thyrotoxicosis. Lancet. 1973 May 26;1(7813):1196–1196. doi: 10.1016/s0140-6736(73)91210-5. [DOI] [PubMed] [Google Scholar]


