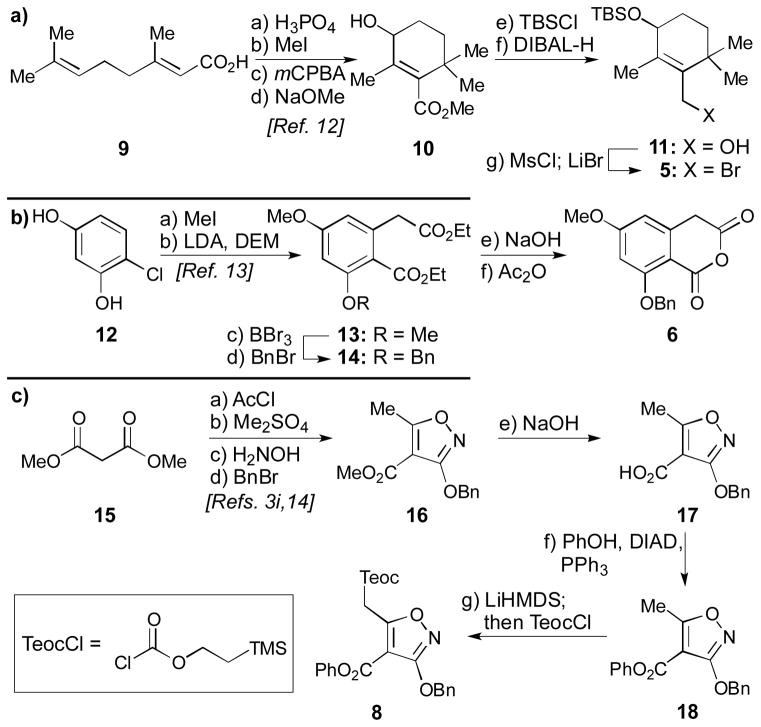
Scheme 1.
a) Synthesis of allylic bromide 5; b) synthesis of cyclic anhydride 6; and c) synthesis of isoxazole 8. Reagents and conditions: a) a) H3PO4 (0.2 equiv), toluene, reflux, 90 min; b) MeI (3.9 equiv), K2CO3 (2.0 equiv), acetone, 25 °C, 15 h; c) mCPBA (1.2 equiv), CH2Cl2, 0→25 °C, 3 h; d) NaOMe (1.5 equiv), MeOH, reflux, 17 h, 70% yield for 4 steps; e) TBSCl (1.6 equiv), imidazole (2.0 equiv), CH2Cl2, 25 °C, 12 h; f) DIBAL-H (2.7 equiv), CH2Cl2, −78→0 °C, 70 min, 91% for 2 steps; g) Et3N (2.0 equiv), MsCl (1.7 equiv), CH2Cl2, −50 °C, 1 h; then LiBr (3.5 equiv), THF, −50→−20 °C, 1 h, quant.; b) a) MeI (4.0 equiv), K2CO3 (8.0 equiv), acetone, reflux, 15 h, 91%; b) NaH (6.0 equiv), DEM (4.0 equiv), THF, 0 °C, 2.5 h; then LDA (1.0 equiv), THF, 0 °C, 3.5 h, 65%; c) BBr3 (1.35 equiv), CH2Cl2, −78→25 °C, 30 min; d) BnBr (1.1 equiv), Ag2O (1.9 equiv), DMF, 25 °C, 15 h, 66% for 2 steps; e) NaOH (27 equiv), H2O:EtOH 5:7, reflux, 15 h; f) Ac2O (1.1 equiv), toluene, reflux, 1 h, 90% for 2 steps; c) a) MgCl2 (1.0 equiv), Et3N (2.0 equiv), AcCl (1.0 equiv), MeCN, 0→25 °C, 23 h, 96%; b) Me2SO4 (1.3 equiv), K2CO3 (1.3 equiv), DMF, 0→25 °C, 17 h, 54%; c) H2NOH•HCl (1.4 equiv), NaOMe (3.1 equiv), MeOH, 0→25 °C, 24 h, 48%; d) BnBr (1.2 equiv), Ag2O (1.5 equiv), DMF, 25 °C, 18 h, 67%; e) NaOH (1.9 equiv), H2O:EtOH 3:10, 25 °C, 3 h, 99%; f) PPh3 (1.05 equiv), PhOH (1.05 equiv), DIAD (1.05 equiv), THF, reflux, 3 h, 78%; g) LiHMDS (2.2 equiv), THF, −78 °C, 30 min; then TeocCl (2.2 equiv), −78 °C, 2 h, 86%. TBS = tert-butyldimethylsilyl, DIBAL-H = diisobutylaluminum hydride, Ms = methanesulfonyl, DEM = diethylmalonate, LDA = lithium diisopropylamide, DMF = dimethylformamide, DIAD = diisopropylazodicarboxylate, PhOH = phenol, THF = tetrahydrofuran, LiHMDS = lithium hexamethyldisilazide, Teoc = 2-(trimethylsilyl)ethoxycarbonyl.