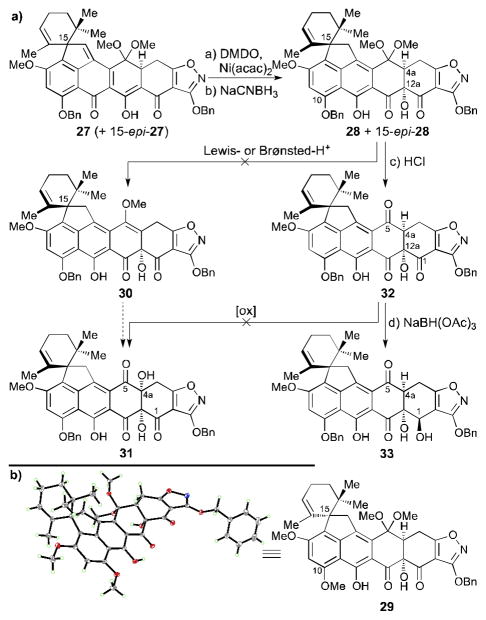
Scheme 3.
a) Unsuccessful attempts to install the C4a hydroxyl group and synthesis of C1 hydroxy compound 33 and b) ORTEP representation of heptacycle 29 (thermal ellipsoids at 30% probability; grey = C, red = O, blue = N, green = H). Reagents and conditions: a) Ni(acac)2 (0.2 equiv), DMDO (5.1 equiv), CH2Cl2, −78→−60 °C, 6.5 h, 36%, 60% brsm, 50% after one recycle; b) NaCNBH3 (10 equiv), THF, −78→−60 °C, 90 min, 39% for 28, 19% for 15-epi-28, chromatographically separated; c) 2 N aq. HCl:THF 1:10, 25 °C, 5 h, quant.; d) NaBH(OAc)3 (1.2 equiv), EtOAc:acetone 1:1, 40 °C, 105 min, 47%; acac = acetylacetonate, DMDO = dimethyldioxirane.