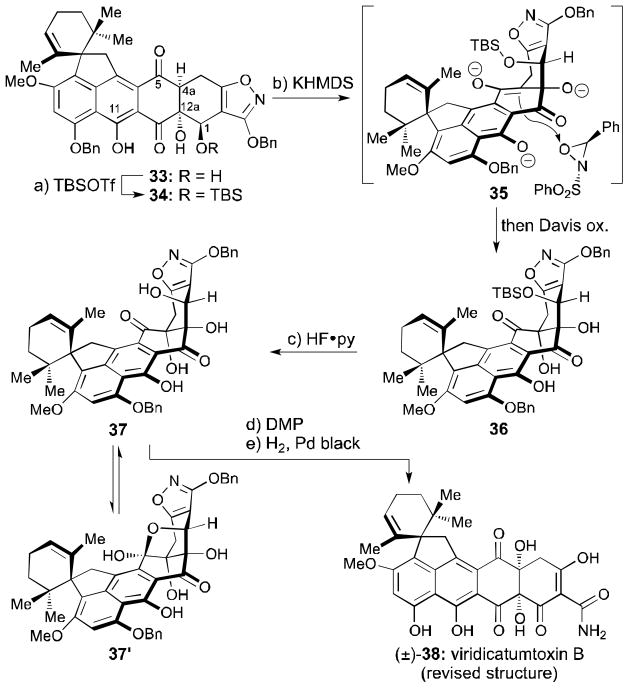
Scheme 4.
C4a oxidation and completion of the synthesis. Reagents and conditions: a) TBSOTf (40 equiv), 2,6-lutidine (60 equiv), CH2Cl2, 0→25 °C, 1 h, 61%; b) KHMDS (3.4 equiv), THF, −78 °C, 1 h; then Davis ox. (3.9 equiv), −78 °C, 1.7 h, 20% + 45% recovered 34; c) HF•py (excess), MeCN, 0→50 °C, 25 h, 61%; d) DMP (3.0 equiv), DCE, 0→50 °C, 7.5 h, 66%; e) H2, Pd black (4.9 equiv), 1,4-dioxane:MeOH 1:1, 25 °C, 8 min, 98%; TBSOTf = tert-butyldimethylsilyl trifluoromethanesulfonate; KHMDS = potassium hexamethyldisilazide, Davis ox. = (±)-trans-2-(phenylsulfonyl)-3-phenyloxaziridine, py = pyridine, DMP = Dess–Martin periodinane, DCE = 1,2-dichloroethane.