Abstract
Exposure of Siberian hamsters to short photoperiod (SD) inhibits ovarian function, including folliculogenesis, whereas function is restored with transfer to long photoperiods (LD). To investigate the mechanism of photostimulated recrudescence, we assessed key folliculogenic factors: anti-Müllerian hormone (AMH), inhibin-α, growth differentiation factor-9 (GDF-9), and bone morphogenic protein-15 (BMP-15) across the estrus cycle and in photoregressed and recrudescing ovaries. Adult hamsters were exposed to either LD or SD for 14 weeks representing functional and regressed ovaries, respectively. Select regressed hamsters were transferred back to LD for two (post-transfer week 2; PTw2) or eight weeks (PTw8). Ovaries were collected and fixed in formalin for immunohistochemistry or frozen in liquid nitrogen for real-time PCR. AMH, inhibin-α, GDF-9 and BMP-15 mRNA and protein were detected in all stages of the estrus cycle. Fourteen weeks of SD exposure increased (p<0.05) ovarian AMH, GDF-9 and BMP-15, but not inhibin-α mRNA levels as compared to LD. Transfer of regressed hamsters to stimulatory long photoperiod for 8 weeks returned AMH and GDF-9 mRNA levels to LD levels levels, and further increased mRNA levels for inhibin-α and BMP-15. Immunostaining of AMH, inhibin-α, GDF-9 and BMP-15 proteins was most intense in preantral/antral follicles and oocytes. The overall immunostaining extent for AMH and inhibin-α generally mirrored mRNA data, though no changes were observed in GDF-9 or BMP-15 immunostaining extent. Shifts in mRNA and protein levels across photoperiod conditions suggest possible syncretic roles for these folliculogenic factors in photostimulated recrudescence via potential regulation of follicle recruitment, preservation and development.
Keywords: seasonal reproduction, folliculogenesis, photostimulation
Introduction
Siberian hamsters (Phodopus sungorus) have adapted to harsh environmental conditions with reduction of reproductive activity during winter months when the days are short and foraging is difficult. Reproductive activity is fully resumed in spring and summer when environmental conditions improve (Reiter, 1980; Glass 1986, Buchanan and Yellon, 1991; Porka-Heiskanan, 1997). Our laboratory, like others (e.g., Schlatt et al. 1993), has reported that adult Siberian hamsters exposed to short days (SD, 8 hours of light per day: 16 hours of dark per day) for sustained periods (14 weeks) reduce reproductive activity, with significant reductions in ovary and uterine mass, antral follicle and corpora lutea numbers, and serum estradiol concentration as compared to those maintained in long photoperiods (LD, 16L:8D) (Moffatt-Blue et. al. 2006). Subsequent transfer of photoinhibited Siberian hamsters to LD for two to eight weeks promotes systemic gonadotropin release leading to ovarian recrudescence: resumption of estrus cyclicity, serum estradiol levels, folliculogenesis and ovulation (Salverson et al. 2008). Return of ovarian function is characterized by resumption of folliculogenesis, a complex process that involves the interaction of pituitary and local gonadotropins with follicle and oocyte-specific factors to guide follicular recruitment and development (reviewed by Trombly et al. 2009). Importantly, the absence of gonadotropins alone does not produce full ovarian regression, nor does the replacement of FSH to SD exposed females fully return the LD ovarian phenotype, suggesting that local ovarian factors beyond gonadotropins play a key role in recrudescence (Zysling et al. 2012).
Members of the TGF-β superfamily have long been implicated as key players in the recruitment, selection, and development of primordial follicles, and the development of primary follicles through ovulation and luteinization (reviewed by Knight and Glister, 2006; Trombly et al. 2009; Myers and Pangas, 2010). Anti-Müllerian hormone (AMH), a homodimeric glycoprotein, is produced in ovarian follicles by granulosa cells of the primary, pre-antral, and small antral, but not primordial follicles (Durlinger et al. 2002a; Durlinger et al. 1999). AMH can constrain follicular development by inhibiting primordial follicle recruitment (preventing their depletion) (Nilsson et al. 2011) and by reducing of FSH sensitivity in growing follicles (reviewed by Knight and Glister 2006; Trombly et al. 2009). Intriguingly, AMH levels also respond to changes in photoperiod; ovarian, but not serum AMH levels are elevated in Siberian hamsters raised in short photoperiods (10L:14D) since birth (Kabithe and Place, 2008; Place and Cruickshank, 2009). Since AMH is critical for regulating follicular development, and is photoresponsive, it is likely to be involved with the return of ovarian function; however, the pattern of AMH mRNA and protein levels during photoperiod induced ovarian recrudescence had not been previously investigated.
Inhibin and activin are gonadal hormones synthesized by granulosa cells of the growing follicles and have opposing functions: activins stimulate pituitary FSH secretion whereas inhibins down-regulate FSH secretion, and both are expressed differentially throughout the estrus cycle (Woodruff et al. 1996; reviewed by Knight and Glister 2006). Inhibin A is a heterodimer of the common α and βA subunits, inhibin B is a dimer of α and βB subunits, and activins are homodimers of the β subunits. FSH stimulates granulosa cell production of all three subunits, and inhibins feed back to inhibit FSH release (reviewed by Bernard et al. 2001). Like AMH, short photoperiod exposure reduces inhibin-α subunit mRNA, inhibin dimeric protein, and all three subunits proteins as compared to long day exposed Siberian hamsters (Kenny et al. 2002a, 2002b). Although these reports infer photoperiod regulation of inhibin secretion, its role in photostimulated recrudescence of regressed ovaries had not been examined.
Growth differentiation factor-9 (GDF-9) and most bone morphogenetic proteins (e.g., BMP-15) are oocyte derived homologous growth factor proteins, and members of the TGF-β superfamily that play important roles in different stages of follicular development and oocyte maturation. GDF-9B was identified as a GDF-9 homolog and named BMP-15 in rat ovaries (Jaitinen et al. 1999). Both GDF-9 and BMP-15 are found in ovaries of mice (McGrath et al. 1995), rats (Dube et al. 1998), humans (Fritzpatrik et al. 1998) and hamsters (Wang and Roy, 2004), and are considered oocyte derived factors that facilitate the growth and maturation of oocytes and inhibit cumulus cell apoptosis (Otsuka et al. 2011; Gilchrist et al. 2008; Sue et al. 2009). While GDF-9 and BMP-15 play crucial roles in oocyte growth and maturation, the effect of photoperiod on GDF-9 and BMP-15 had not been explored.
In Siberian hamsters, short photoperiods severely inhibit reproductive function, which gradually resumes with transfer to photostimulating conditions. We hypothesized that alterations in photoperiod stimulation would influence both mRNA and protein levels of AMH, inhibin-α and oocyte derived growth factors GDF-9 and BMP-15, and that these changes may play a role in ovarian recrudescence in Siberian hamsters.
Results
Effect of photoperiod on ovary weight, serum estradiol levels and follicle count
Consistent with previous studies, exposure to 14 weeks of short day (SD; 8L:16D) reduced both ovarian weight and serum estradiol levels as compared to females exposed to LD photoperiod for the same duration (p<0.05 for both measures; Table 1). In contrast, photostimulation (16L: 8D) of regressed females for eight weeks (PTw8) restores both ovarian mass and estradiol concentrations to levels no different than LD (Table 1). Follicle development and ovulation occurs regularly in LD stimulated females; however, is also severely inhibited by SD exposure: no antral follicles or corpora lutea are noted in SD females (Table 2). In addition, a significant increase in hypertrophied ‘luteinized’ atretic follicles, characteristic of regressed Siberian hamster ovaries, was noted in SD as compared to LD controls (p<0.05; Table 2). Eight weeks of photostimulation restored folliculogenesis, with numbers of antral follicles and CL no different from LD controls (p>0.05; Table 2). To provide background and clarity for the present results, data in Tables 1 and 2 (other than PTw2 and luteinized atretic follicles) have been partially published before in a sister study (Shahed and Young, 2011). All other data represent novel findings.
Table 1.
Ovarian mass and estradiol production are affected by photoperiod exposure.
| LD | SD | PTw2 | PTw8 | |
|---|---|---|---|---|
| Ovary weight (mg) | 13.18 ± 1.8a | 6.01 ± 1.0b | 11.84 ± 1.6a | 12.20 ± 0.7a |
| Serum estradiol (pg/ml) | 85.99 ± 13.5a | 27.12 ± 2.8b | 42.89 ± 15b | 53.27 ± 11a |
Results are expressed as mean ± SEM. A subset of these data (LD, SD and PTw8) has been previously published in a companion study (Shahed and Young 2011).
Table 2.
Photoperiod exposure affects dominant ovarian structures.
| Ovarian Structure | LD | SD | PTw2 | PTw8 |
|---|---|---|---|---|
| Preantral | 5.6 ± 1.4a | 1.93 ±0.26b | 5.25 ± 1a | 5.16 ± 0.8a |
| Antral Follicles | 0.87 ± 0.2a | 0.12 ± 0.06b | 0.50 ± 0.1a | 1.26 ± 0.2a |
| Corpora lutea | 2.25 ± 0.46a | 0.04 ± 0.04b | 0.17 ± 0.1b | 2.15 ±0.26a |
| Lutenized atretic follicles | 0.27 ± 0.12a | 6.05 ± 1.2b | 1.08 ± 0.4a | 0.75 ± 0.2a |
Ovarian sections (n=5-6 hamsters/group, 3 section/slide) were stained with eosin and hemotoxylin and follicles were counted. A subset of these data (PA, A, and CL data for LD, SD and PTw8) has been previously published in a companion study (Shahed and Young 2011).
AMH mRNA levels and protein immunostaining
Estrus cycle
AMH mRNA was detectible in all stages of the estrous cycle, but was highest in proestrus (P) compared to the estrus (E), diestrus I (DI), and diestrus II (DII) stages (p<0.05; Figure 1a). AMH immunostaining was also detected in all stages of the estrus cycle, with high levels of immunodetection noted predominantly in preantal and antral follicles (Table 3). AMH immunostaining intensity peaked in antral follicles during P as compared to the DII stage (p<0.05; Table 3). No differences in immunostaining intensity were noted for other ovarian structures, and overall relative extent of immunostaining did not change significantly across estrus cycle stages (p>0.05; Table 3).
Figure 1. Ovarian AMH.
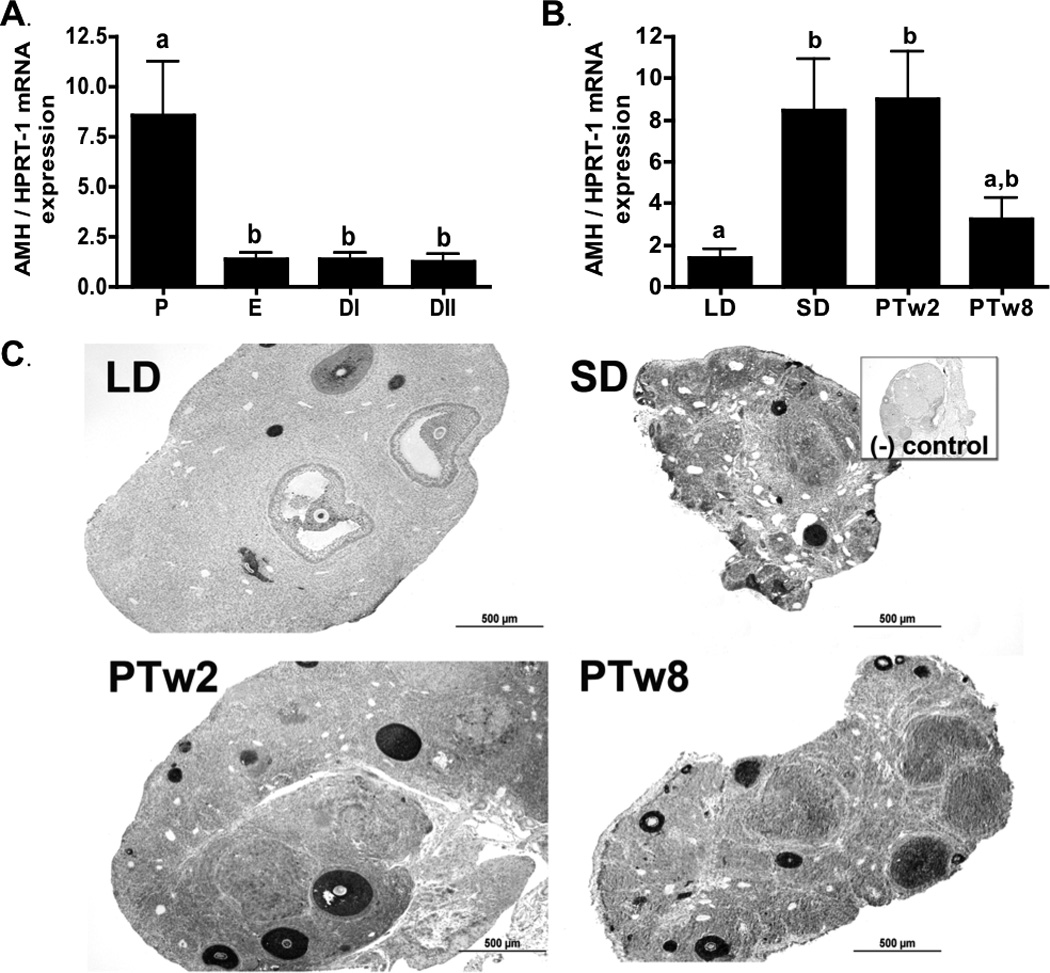
1a) Ovarian AMH mRNA levels during the estrus cycle. P (proestrus), E (estrus), DI (diestrus I) and DII (diestrus II). 1b) Ovarian AMH mRNA levels during photoperiod induced regression/recrudescence. LD= long day exposure/control, SD= inhibitory short day exposure, PTw2= post transfer from SD to LD for 2 weeks, PTw8= post transfer from SD to LD for 8 weeks. 1c) AMH immunostaining during photoperiod induced regression /recrudescence. Representative immunostaining histographs of ovarian sections from each photoperiod group are shown. Inset depicts negative control processed without primary antibody. All graphical results are presented as mean ± SEM, and groups with different letters are significantly different (p<0.05).
Table 3.
Average relative immunostaining index across ovarian structures, and overall immunostaining extent in the estrous cycle.
| Proestrus | Estrus | Diestrus I | Diestrus II | |
|---|---|---|---|---|
| AMH | ||||
| Preantral | 3.68 ± .2 | 3.67 ± 0.2 | 3.28 ± 0.5 | 2.67 ± .6 |
| Antral | 3.2 ± 0.4 a | 2.53 ± 0.7 a,b | 2.27 ± 0.5 a,b | 0.93 ± 0.4 b |
| Corpora lutea | --- | 1.73 ± 0.5 | 1.07 ± 0.7 | 0.20 ± 0.1 |
| Stroma CT | 0.36 ± 0.1 | 0.53 ± 0.1 | 0.45 ± 0.0 | 0.13 ± 0.1 |
| Stroma Ste | 1.20 ± 0.4 | 1.47 ± 0.7 | 1.60 ± 0.4 | 0.40 ± 0.2 |
| Overall Extent | 2.70 ± 0.2 | 3.07 ± 0.6 | 2.27 ± 0.7 | 1.80 ± 0.6 |
| Inhibin-α | ||||
| Preantral | 1.60 ± 0.5 | 2.80 ± 0.5 | 2.70 ± 0.2 | 2.10 ± 0.7 |
| Antral | 1.90 ± 0.7 a | 2.30 ± 0.2 a | 0 ± 0 b | 1.1 ± 0.4 a,b |
| Corpora lutea | --- | 0.2 ± 0.2 | 0 ± 0 | 0 ± 0 |
| Stroma CT | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| Stroma Ste | 0.42 ± 0.2 | 0.13 ± 0.1 | 0 ± 0 | 0.13± 0.1 |
| Overall Extent | 1.62 ± 0.32 a,b | 2.66 ± 0.15a | 0.89 ± 0.22b | 1.24 ± 0.5b |
| GDF-9 | ||||
| Preantral | 0.90 ± 0.1 | 0.95 ±0.2 | 1.15 ± 0.5 | 0.43 ± 0.1 |
| Antral | 0.66 ± 0.1 | 0.72 ± 0.1 | 0.10 ± 0.1 | 0.23 ± 0.2 |
| Corpora lutea | --- | 1.14 ± 0.1 a,b | 1.35 ± 0.4a | 0.12 ± 0.1b |
| Stroma CT | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| Stroma Ste | 0.93 ± 0.3 | 0.95 ± 0.1 | 1.11 ± 0.4 | 0.41 ± 0.2 |
| Overall Extent | 1.13 ± .1 | 1.09 ± 0.3 | 1.52 ± 0.5 | 0.28 ± 0.1 |
| BMP-15 | ||||
| Preantral | 2.17 ± 0.4 a,b | 2.67 ± 0.4 a,b | 3.21 ± 0.2 a | 2.00 ± 0.1 b |
| Antral | 2.66 ± 0.4 | 3.06 ± 0.5 | 3.00 ± 0.5 | 2.27 ± 0.2 |
| Corpora lutea | --- | 1.68 ± 0.9 | 2.23 ± 0.2 | 1.45 ± 0.3 |
| Stroma CT | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| Stroma Ste | 1.50 ± 0.5 | 1.75 ± 0.2 | 1.42 ± 0.4 | 1.06 ± 0.5 |
| Overall Extent | 3.00 ± 0.6 | 3.14 ± 0.3 | 3.29 ± 0.3 | 2.57 ± 0.2 |
Average relative immunostaining intensity by ovarian structure and average overall extent of immunostaining across ovarian cross sections (n=3–6 per animal, 4–6 animals per group) for: AMH, Inhibin α, GDF-9 and BMP-15 following during the estrus cycle. Both immunostaining intensity and extent were scored on a scale of 0–4. Stroma CT= stroma primarily consisting of connective tissue, Stroma Ste= stroma including potential steroidogenic interstitial glands. Values represented as mean (SEM). Groups with different letters are significantly different (p < 0.05).
Photoperiod induced regression/recrudescence
AMH mRNA levels were 4-fold higher in SD ovaries as compared to levels in LD control hamsters (p<0.05; Figure 1b). Photostimulation of regressed hamsters for two weeks (PTw2) maintained high AMH mRNA levels as compared to LD (p<0.05). In contrast, AMH mRNA levels in the PT hamsters were not different from LD controls after eight weeks (p>0.05; Figure 1b).
AMH immunostaining intensity in LD ovaries was most prominent in preantral (PA) and early antral (A) follicles, with lower levels of staining detected in corpora lutea (CL), stromal connective tissue, and steroidogenic stroma in LD controls (p<0.05; Table 4). While staining was most intense in preantral follicles, AMH immunodetection was also robust in luteinized granulosa cells of atretic follicles that are characteristic of SD ovaries (Figure 1c), leading to the peak of intensity noted in steroidogenic stroma among SD and PT ovaries as compared to LD (p<0.05; Table 4). In antral follicles, AMH staining intensity peaked in PTw2 as compared to LD and PTw8 (p<0.05); similarly, the relative overall extent of AMH immunostaining across the ovarian sections also peaked in PTw2 as compared to LD controls (p <0.05; Table 4).
Table 4.
Average relative immunostaining index across ovarian structures, and overall immunostaining extent with different photoperiod exposure.
| LD | SD | PTw2 | PTw8 | |
|---|---|---|---|---|
| AMH | ||||
| Preantral follicles | 3.87 ± 0.1 | 3.07 ± 0.5 | 3.52 ± 0.5 | 2.87 ± 0.6 |
| Antral follicles | 1.17 ± 0.4a | --- | 3.00 ± 0.4b | 1.67 ± 0.4a |
| Corpora lutea | 0.57 ± 0.4 | --- | 1.10 ± 0.3 | 0.87 ± 0.2 |
| Stroma CT | 0.19 ± 0.1 | 0.56 ± 0.3 | 0.73 ± 0.2 | 0.33 ± 0.1 |
| Stroma Ste | 0.20 ± 0.1 a | 1.37 ± 0.5 b | 1.51 ± 0.1 b | 0.73 ± 0.2 a,b |
| Overall Extent | 1.39 ± 0.3 a | 2.56 ± 0.4 a,b | 3.29 ± 0.4 a | 2.67 ± 0.4 a,b |
| Inh α | ||||
| Preantral follicles | 3.11 ± 0.4a | 1.70 ± 0.1b | 3.00 ± 0.5a | 3.90 ± 0.3a |
| Antral follicles | 3.83 ± 0.1 | --- | 3.50 ± 0.3 | 3.42 ± 0.3 |
| Corpora lutea | 0 ± 0 | --- | 0 ± 0 | 0 ± 0 |
| Stroma CT | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| Stroma Ste | 0 ± 0 | 0 ± 0 | 0.29 ± 0.1 | 0.04 ± 0 |
| Overall Extent | 1.67 ± 0.4 a | 0.70 ± 0.1 b | 2.66 ± 0.1 c | 2.55 ± 0.3 c |
| GDF-9 | ||||
| Preantral follicles | 2.51 ±0.2 a | 1.62 ± 0.2 b | 2.39 ± 0.1a,b | 1.61 ± 0.2 b |
| Antral follicles | 2.75 ± 0.3 | --- | 3.04 ± 0.3 | 1.94 ± 0.2 |
| Corpora lutea | 2.59 ± 0.2a | --- | 2.99 ± 0.5a | 1.43 ± 0.1b |
| Stroma CT | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| Stroma Ste | 0.72 ± 0.3 | 1.63 ± 0.3 | 1.79 ± 0.6 | 1.08 ± 0.3 |
| Overall Extent | 2.96 ± 0.4 | 2.81 ± 0.3 | 3.13 ± 0.3 | 2.75 ± 0.2 |
| BMP-15 | ||||
| Preantral follicles | 1.12 ± 0.4 | 1.23 ± 0.3 | 1.30 ±0.3 | 1.22 ± 0.4 |
| Antral follicles | 1.33 ± 0.3 | --- | 1.32 ± 0.1 | 1.30 ± 0.2 |
| Corpora lutea | 1.08 ± 0.2 | --- | 1.50 ± 0.2 | 1.79 ± 0.4 |
| Stroma CT | 0.25 ± 0.1 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| Stroma Ste | 0.72 ± 0.3 | 1.63 ± 0.3 | 1.79 ± 0.6 | 1.07 ± 0.4 |
| Overall Extent | 2.30 ± 0.3 | 2.25 ± 0.4 | 2.19 ± 0.4 | 2.29 ± 0.4 |
Average relative immunostaining intensity by ovarian structure and average overall extent of immunostaining across ovarian cross sections (n=3–6 per animal, 4–6 animals per group) for: AMH, Inhibin α, GDF-9 and BMP-15 following different photoperiod exposures. Both immunostaining intensity and extent were scored on a scale of 0–4. Stroma CT= stroma primarily consisting of connective tissue, Stroma Ste= stroma including potential steroidogenic interstitial glands. Values represented as mean (SEM). Groups with different letters are significantly different (p < 0.05).
Inhibin-α mRNA levels and protein immunostaining
Estrus cycle
Inhibin-α mRNA was present in all stages of the estrous cycle; however, relative levels were significantly higher during E, DI, and DII as compared to P (Figure 2a). Inhibin-α protein immunoreactivity was also observed throughout the estrous cycle. While immunostaining intensity did not change across estrus cycle stages in preantral follicles, CL, and stromal tissues (all p>0.05; Table 3), inhibin-α intensity did peak in antral follicles in P and E as compared to DI (p<0.05; Table 3). The average overall extent of inhibin-α immunolocalization across ovarian cross sections peaked in E as compared DI and DII (p<0.05; Table 3).
Figure 2. Ovarian Inhibin-α.
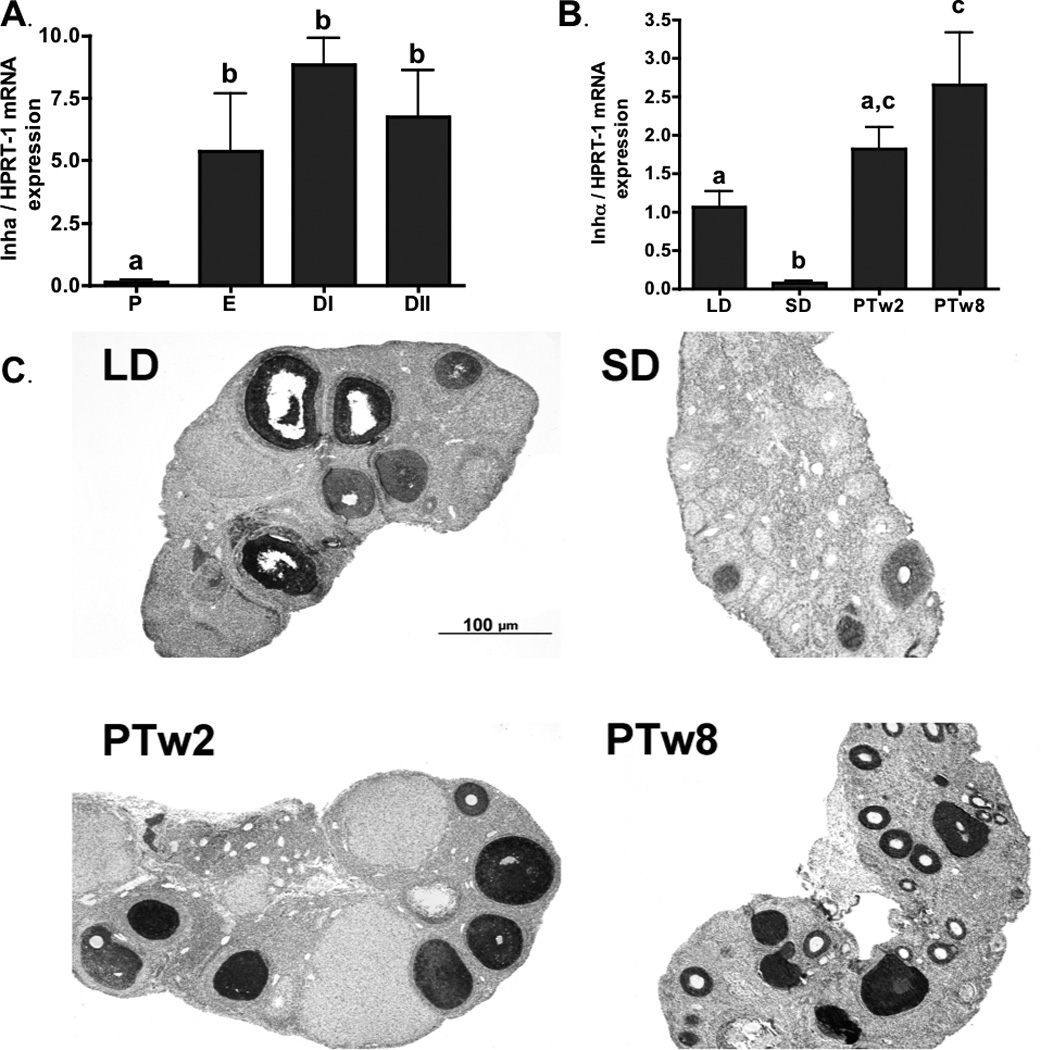
2a) Ovarian Inhibin-α mRNA levels during the estrus cycle. P (proestrus), E (estrus), DI (diestrus I) and DII (diestrus II). 2b) Ovarian Inhibin-α mRNA levels during photoperiod induced regression/recrudescence. LD= long day exposure/control, SD= inhibitory short day exposure, PTw2= post transfer from SD to LD for 2 weeks, PTw8= post transfer from SD to LD for 8 weeks. 2c) Inhibin-α immunostaining during photoperiod induced regression /recrudescence. Representative immunostaining histographs of ovarian sections from each photoperiod group are shown. Inset depicts negative control processed without primary antibody. All graphical results are presented as mean ± SEM, and groups with different letters are significantly different (p<0.05).
Inhibin-α during regression/recrudescence
Inhibin-α mRNA was present in all groups: LD, SD, PTw2 and PTw8 (Figure 2b). Fourteen weeks of SD exposure reduced inhibin-α mRNA levels in SD as compared to LD controls (p<0.05; Figure 2b). Subsequent transfer of regressed hamsters to LD increased intra-ovarian inhibin-α mRNA, reaching higher relative levels in PTw8 as compared to both SD and LD (p<0.05; Figure 2b).
Immunostaining for the inhibin-α protein was primarily detected in preantral and antral follicles, along with very reduced amounts in CL tissue, and no detection in the stroma (Figure 2c). The intensity of preantal follicle immunostaining dropped significantly in SD as compared to the LD and PT groups (p<0.05; Table 4), whereas no changes were noted across the LD and PT ovaries for intensity of immunostaining in antral follilces (p>0.05; Table 4). The average overall extent measurements of inhibin-α immunostaining, like the mRNA, were significantly lower in SD compared to LD controls, but returned to LD levels with photostimulation in the PTw2 and PTw8 groups (p< 0.05; Table 4).
Growth and differentiation factor-9 (GDF-9) and bone morphogenic protein 15 (BMP-15) mRNA levels and protein immunostaining
GDF-9 and BMP-15 in the estrus cycle
GDF-9 and BMP-15 mRNA was present throughout the estrous cycle. GDF-9 mRNA levels were lowest in P as compared to the E, DI, and DII stages (p<0.05; Figure 3a). Showing a slightly different pattern, BMP-15 mRNA levels peaked in E as compared to in P (p<0.05) but were not different than DI and DII (Figure 4a). Like mRNA, immunostaining for GDF-9 and BMP-15 proteins was detected in all stages of the estrus cycle, although no differences were noted in average overall extent across stages for either protein (p>0.05; Table 3). Immunostaining intensity for both GDF-9 and BMP-15 showed peaks in DI, with GDF-9 intensity higher in DI than DII in corpora lutea, and BMP-15 intensity higher in DI as compared to DII in preantral follicles (p<0.05; Table 3).
Figure 3. Ovarian GDF-9.
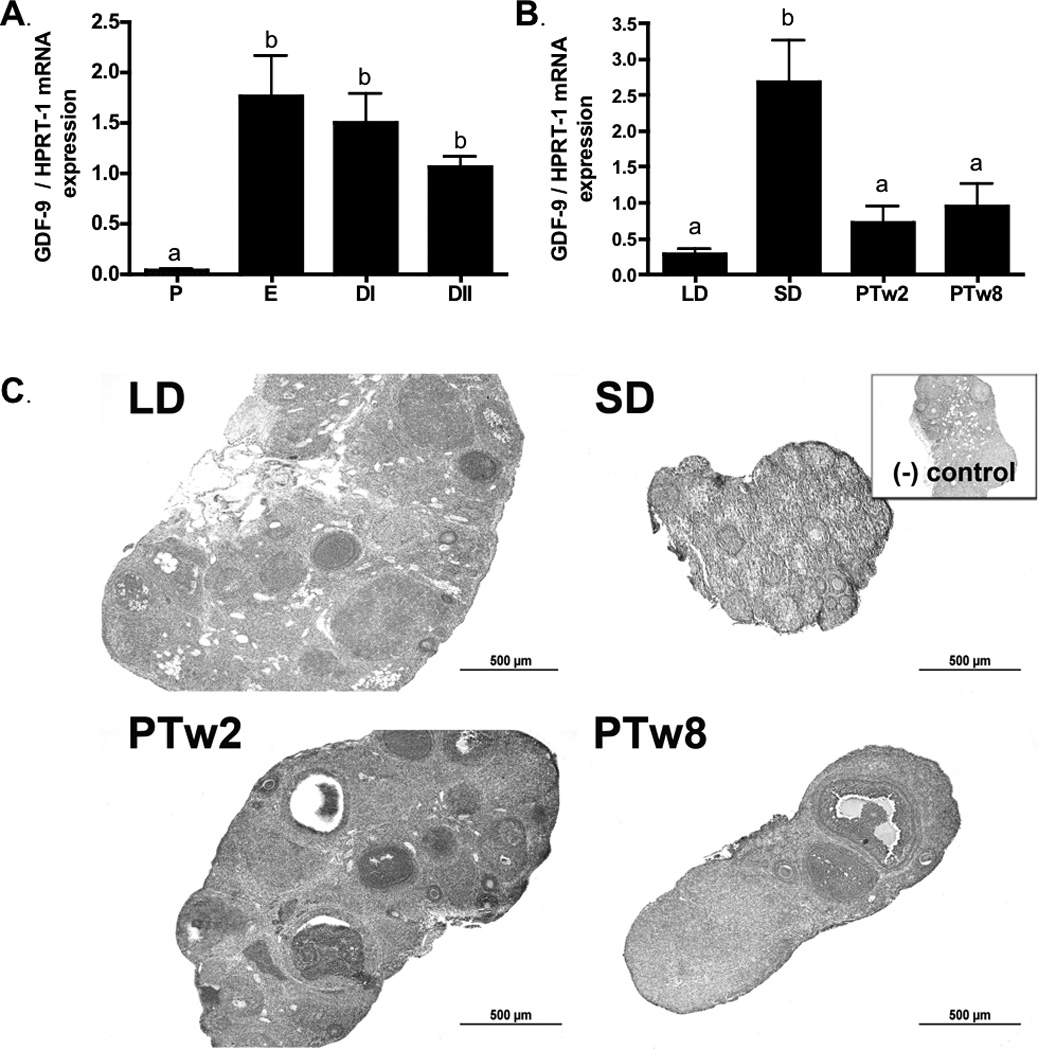
3a) Ovarian GDF-9 mRNA levels during the estrus cycle. P (proestrus), E (estrus), DI (diestrus I) and DII (diestrus II). 3b) Ovarian GDF-9 mRNA levels during photoperiod induced regression/recrudescence. LD= long day exposure/control, SD= inhibitory short day exposure, PTw2= post transfer from SD to LD for 2 weeks, PTw8= post transfer from SD to LD for 8 weeks. 3c) GDF-9 immunostaining during photoperiod induced regression /recrudescence. Representative immunostaining histographs of ovarian sections from each photoperiod group are shown. Inset depicts negative control processed without primary antibody. All graphical results are presented as mean ± SEM, and groups with different letters are significantly different (p<0.05).
Figure 4. Ovarian BMP-15.
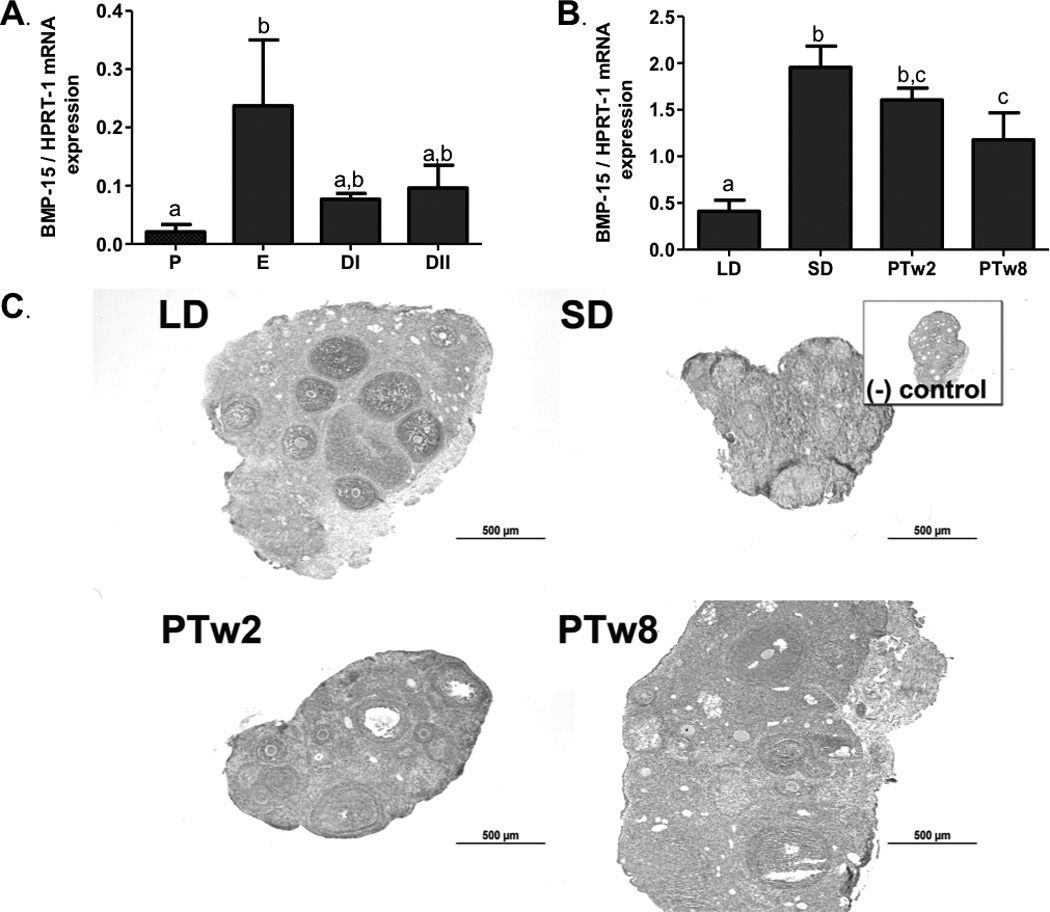
4a) Ovarian BMP-15 mRNA levels during the estrus cycle. P (proestrus), E (estrus), DI (diestrus I) and DII (diestrus II). 4b) Ovarian BMP-15 mRNA levels during photoperiod induced regression/recrudescence. LD= long day exposure/control, SD= inhibitory short day exposure, PTw2= post transfer from SD to LD for 2 weeks, PTw8= post transfer from SD to LD for 8 weeks. 4c) BMP-15 immunostaining during photoperiod induced regression /recrudescence. Representative immunostaining histographs of ovarian sections from each photoperiod group are shown. Inset depicts negative control processed without primary antibody. All graphical results are presented as mean ± SEM, and groups with different letters are significantly different (p<0.05).
GDF-9 and BMP-15 during regression/recrudescence
GDF-9 mRNA was present throughout the photoperiod exposure groups. GDF-9 mRNA levels were higher in SD as compared to LD (p<0.05), an increase subsided once females were transferred to photostimulating conditions; mRNA returned to LD control levels in both PTw2 and PTw8 ovaries (Figure 3b). BMP-15 mRNA levels responded slightly differently; while they increased with SD compared to LD exposure, they remained high in PTw2 and PTw8 ovaries as compared to LD (p<0.05; Figure 4b).
GDF-9 and BMP-15 immunostaining showed a similar pattern and was detected in all ovaries regardless of photoperiod exposure (Figures 3c and 4c). Both GDF-9 and BMP-15 overall immuostaining intensity was more intense in PA and A follicles and CL tissue, as compared to stromal tissues (p< 0.05; Figure 3d, 4d), with particularly high immunostaining intensity observed in oocytes and granulosa cells for both proteins.
GDF-9 immunostaining was detected in the ovaries of all photoperiod groups, although it showed no significant differences in overall extent between groups (p> 0.05; Table 4). Immunostaining of GDF-9 was predominantly localized to pre-antral follicles, antral follicles, and corpora lutea, with a moderate amount in steroidogenic stroma, and no staining noted in connective tissue-based stroma (Figure 3c). The intensity of GDF-9 immunostaining did differ between groups in preantal follicles and corpora lutea, with preantral follicles in LD ovaries showing higher intensity staining than preantral follicles in SD and PTw8 (p<0.05; Table 4), and corpora lutea staining intensity higher in LD and PTw2 as compared to PTw8 (p<0.05; Table 4). BMP-15 protein was also detected in all photoperiod groups, although no differences were noted in individual intensities or average overall extent of staining across the ovarian sections (p>0.05; Table 4).
Discussion
Because members of the TGF-β superfamily play crucial roles in folliculogenesis, this study investigated the mRNA and protein levels of follicular growth factors (AMH, inhibin-α, GDF-9 and BMP-15) during photostimulated ovarian recrudescence. These factors act at different stages of follicular growth and appear to be differentially regulated during the return to ovarian function. We show, for the first time, that exposing adult Siberian hamsters to 14 weeks of short photoperiod significantly increases ovarian AMH, GDF-9 and BMP-15, and decreases inhibin-α mRNA as compared to LD. Additionally, immunostaining of AMH, inhibin-α, GDF-9, and BMP-15 proteins show differential staining during the estrous cycle and photoperiod induced regression/ recrudescence in Siberian hamsters.
In the present study, ovarian AMH mRNA and protein peaked in the proestrus stage of the estrous cycle compared to other stages (Figure 1a). This differs from what has been reported in postnatal rats, with no changes noted in the in situ detection of AMH and AMHIIR mRNA, except a reduction in preantral follicles during estrus, as compared to other stages (Baarends et al. 1995). However, since levels were highest in small preantral follicles, it follows that AMH mRNA might peak when these follicles are plentiful in the ovary, such as during the proestrus and estrus stages, where the number of preantral follicles is highest in Siberian hamsters (Vrooman and Young, 2011). In the present study, AMH immunostaining was highest in granulosa cells of primary, preantral and antral follicles with very little in CLs (Figure 1c). This pattern is consistent with previous reports in Siberian hamster (Kabithe and Place 2008), mouse (Baarends et al. 1995), human, and rat ovaries (Weenen et al. 2004).
Ovarian AMH mRNA and protein levels were also altered with exposure to different photoperiods in Siberian hamsters. While short photoperiod exposure reduces ovarian function, it increases AMH mRNA levels as compared to LD ovaries (Figure 1b). These results are similar to a previous report, where Siberian hamsters raised under SD conditions (10L:14D) from birth showed increased levels of AMH protein as compared to those raised in LD (16L:8D) (Kabithe and Place, 2008). In contrast, these results differ from a recent report showing no changes in ovarian AMH mRNA in Siberian hamsters raised in SD conditions for 7.5 weeks postnatally (Zysling et al. 2012). This difference may, in part, be related to experimental design, as the current study used adult hamsters instead of neonates and the SD exposure time was longer (14 weeks). An increase in AMH in SD ovaries is consistent with its role as an inhibitor of both follicle growth and primordial follicle recruitment (McGee and Hseuh, 2000; Durlinger et al. 2002). While the lack of gonadotropin support provides a central signal to initiate gonadal regression, a local increase in AMH in the ovary may assist in maintaining gonadal quiescence. When AMH is knocked out in mice, the ovaries respond with more preantral and small antral follicles (Durlinger et al. 1999); such recruitment and growth is in direct opposition of the cessation of folliculogenesis observed in ovarian atrophy. Interestingly, the levels of both AMH mRNA and protein remain high during the first weeks of recrudescence, not returning to LD levels until 8 weeks of photostimulation, when ovarian steroidogenesis and folliculogenesis fully recover (Figure 1b; Table 4). The persistence in increased AMH may be related to its inhibitory effect on FSH stimulated follicle growth and in maintaining primordial pool numbers (reviewed by Durlinger et al. 2002); as it would be critical to restrict the number of growing follicles during the rapid resurgence of FSH during recrudescence. Taken together, it is likely that the SD rise in AMH is involved with maintaining the primordial pool and limiting photostimulated follicular growth in Siberian hamsters.
Inhibins, also members of TGFβ superfamily, are critical players in folliculogenesis that are both stimulated by and negatively regulate FSH (Bernard et al. 2001). In Siberian hamsters, levels of both inhibin-α subunit mRNA and protein immunostaining were constant through most of the estrus cycle, declining only in proestrus (Figure 2a), and were higher in growing follicles as compared to CL (Figure 2c, Tables 3, 4). In rats, inhibin-α subunit mRNA also varies across the estrus cycle, declining at times when FSH concentrations increase, and peaking on the evening of proestrus (Meunier et al. 1988; Murata et al. 1997; Woodruff et al. 1996). Timing of sample collection and species difference may account for this difference in results, as ovaries in the present study were collected after vaginal cytology was assessed, but timing of estrus stages was not synchronized.
Inhibin-α has been used as a marker of follicular growth (reviewed by Knight and Glister, 2006), which is consistent with the results of the present study where inhibin-α levels were high in functional or recrudescing ovaries and declined with SD induced ovarian atrophy (Figure 2b, Table 4). These results concur with the decreases in inhibin-α, but not inhibin-βa and -βb subunit mRNA levels in that occur in SD exposed Siberian hamster ovaries (Kenny et al. 2002a), and with the decline in inhibin immunostaining observed during SD induced testicular atrophy in male Siberian hamsters (Culler et al. 1991). In contrast, testicular inhibin-α increases in male Siberian hamsters raised in SD (Rao et al. 1995), as does inhibin-like activity in Sertoli cell cultures from regressed (optically enucleated) golden hamsters (Berkowitz and Heindel, 1987). While the lower inhibin-α levels in SD regressed ovaries may simply reflect the lack of follicles beyond the preantral stage (Table 2), it may be that the reduced levels observed in SD preantral follicles may serve to limit initial feedback to FSH secretion as the ovary resumes function during recrudescence (Table 4). During photostimulated recrudescence, inhibin-α subunit mRNA and extent of protein immunoreactivity increased significantly as compared to both LD and SD groups (Figure 2b, Table 4). These data correspond to increases observed in inhibin-α mRNA levels following a gonadotropin challenge in LD exposed female Siberian hamsters (Kenny et al. 2002b), as recrudescence follows the return of gonadotropin secretion. Interestingly, the response to gonadotropin stimulation was blunted in SD exposed females as compared to LD (Kenny et al. 2002b), which may explain the staggered increase in ovarian inhibin-α observed in recrudescing groups in the current study (Figure 2b).
Growth differentiation factor-9 (GDF-9) and bone morphogenic protein-15 (BMP-15) are members of the TGFβ superfamily produced by oocytes that play species-specific cooperative roles in functional ovaries (Yan et al. 2001). GDF-9 and BMP-15 are present during all stages of follicular growth, and are likely released into the follicular fluid to act on granulosa cells (Silva et al. 2005; Jaywardana et al. 2006; Sun et al. 2010; Otsuka et al. 2011; Motterhead et al. 2012). Both GDF-9 and BMP-15 receptor II are expressed in Siberian hamster ovaries, with GDF-9 mRNA critical for primordial follicle growth (Wang and Roy 2006, 2009). We investigated changes of these oocyte-derived factors during the estrus cycle in Siberian hamsters, finding that both GDF-9 and BMP-15 mRNA and protein were present in all stages of the cycle, and that their mRNA, but not protein levels (Figure 3, 4, Table 3), declined in proestrus as compared to all other stages. While BMP-15 mRNA levels change with the stage of follicular development in rats, no apparent changes were noted across the stages of the estrous cycle (Ericson and Shimasaki, 2003), although levels of both GDF-9 and BMP-15 mRNA decline as follicles reach the antral stage in mice (Monti and Redi, 2009). Intense GDF-9 and BMP-15 immunostaining was localized primarily to the oocytes of preantral and antral follicles in the present study; however, some staining was also detected in granulosa cells (Figure 3c, 4c). These data correlate with localization patterns observed in mice, pigs, and goats, where GDF-9 and BMP-15 protein is localized to oocytes and granulosa cells, while other studies demonstrate immunoreactivity in CL of humans and goats (Sun et al. 2010; Silva et al. 2005). In contrast, in 15-day old Siberian hamsters, GDF-9 immunostaining localizes exclusively to oocytes (Wang and Roy, 2006). This apparent difference may be due to the fact that hamsters used in the present study were adults more than 20 weeks old, and it is likely that developmental changes alter patterns of GDF-9 localization.
While GDF-9 and BMP-15 mRNA were expressed in Siberian hamster ovaries regardless of photoperiod exposure, mRNA levels peaked with 14 weeks of SD exposure (Figures 3b and 4b). These increased levels were similar to results observed with AMH, and it suggests that these genes may be critical to maintain follicles within the photoregressed ovary. Both GDF-9 and BMP-15 promote follicular survival by suppressing granulosa cell apoptosis and thus reducing follicular atresia (Hussein et al. 2005; Orisaka et al. 2006). Transferring reproductively regressed females to LD photoperiods returned GDF-9 mRNA to levels observed in the LD controls (Figure 3b); however, this was not the case for BMP-15 mRNA which remained at high levels despite weeks of photostimulation (Figure 4b). The decrease in GDF-9 may be related to its role in granulosa cell stimulation; limiting proliferation and follicle growth during initial return to ovarian function might be necessary to prevent an excessive response to initial resumption of FSH stimulation. Examination of the protein levels during photoperiod changes revealed decreases in the intensity of GDF-9 immunoreactivity within preantral follicles during SD exposure, which reverses with photostimulation (Table 4). In contrast, no changes were noted in staining intensity in other ovarian structures or in the extent of GDF-9 immunostaining across photoperiod exposure groups, and no changes in protein levels were noted in any structure for BMP-15 (Table 4). The discrepancy between these results and mRNA levels may be in the quantification method: scoring of immunohistochemistry patterns provides relative and semi-quantitative data, and while they give additional insight into protein changes, do not reflect the more quantitative whole-ovary mRNA analysis afforded by real time PCR.
Folliculogenesis is a complex process in which endocrine, ovarian, follicle, and oocyte derived growth factors play key and cooperative roles (Yan et al. 2001). Adding to the complexity of folliculogenesis is the process of photoperiod induced ovarian regression and recrudescence. While this process is centrally regulated by gonadotropins, inhibition of GnRH and replacement of FSH alone do not fully mimic photoperiod mediated ovarian regression and recrudescence (Zysling et al. 2012), suggesting that local intra-ovarian factors are critical to these processes. Our results show that AMH, inhibin-α, GDF-9 and BMP-15 are altered differentially during photoperiod induced regression and recrudescence. In photoregressed ovaries, AMH, GDF-9 and BMP-15, but not inhibin-α, mRNA levels were higher than ovaries from LD exposed females. Since SD exposure reduces plasma FSH concentrations that promote inhibin synthesis, the lower inhibin-α mRNA levels in SD ovaries follow the expected pattern. Although GDF-9 and BMP-15 promote development of normal ovarian follicles, an increase in the levels of these factors along with increases in AMH in SD ovaries is interesting and consistent with roles in follicular preservation via their anti-apoptotic properties (GDF-9 and BMP-15), and limitation of primordial follicle recruitment (AMH). Photostimulated recrudescence of the ovary appears to be a process that differs from typical LD ovarian function and from gonadal development/maturation. While AMH and GDF-9 mRNA return to LD levels with post transfer photostimulation, both inhibin-α and BMP-15 levels increased. These results stress to the need for continued study of the complex process of photostimulated recrudescence, as the return to ovarian function appears to occur via pathways that may differ from those that regulate normal ovarian function.
Methods and Materials
Animals
Female Siberian hamsters (Phodopus sungorus, > 60 days of age) were obtained from the breeding colony of Dr. Katherine Wynne-Edwards, Queen’s University (Kingston, Ontario, Canada) and were housed individually at 20 ± 2°C, in our AAALAC-approved facilities and received food and water ad libitum. All experiments were conducted as per California State University Long Beach and NRC guidelines for use of laboratory animals, and with specific project approval from our Institutional Animal Care and Use Committee.
Estrous cycle experiment
To stimulate and help synchronize the estrous cycle, four male hamsters were placed in the same room as the females and soiled male bedding was placed into individual female cages prior to tissue collection (Moffatt-Blue et al., 2006, Dodge et al., 2002). Ovaries were initially staged in proestrus (P), by estrus (E), diestrus I (DI), and diestrus II (DII) by vaginal cytology as described previously (Moffatt-Blue et al., 2006) and stages later confirmed with ovarian morphology. Hamsters (n=4–5/group) were anesthetized (ketamine [200 mg/kg] plus xylazine [20 mg/kg]) and euthanized via cervical dislocation. Ovaries were removed, and one was frozen in liquid nitrogen (qPCR analysis) and the contralateral gonad was fixed in 4% buffered formalin (follicle counts and immunohistochemistry).
Regression/recrudescence experiment
A portion of these hamsters represent a subset from a previously published study examining different ovarian factors (Shahed and Young, 2011). Briefly, four groups of hamsters were exposed to long days (LD, 16L:8D) or short days (SD, 8L:16D) as follows: LD group (n=5) 14 weeks in LD; SD group (n=6) 14 weeks in SD; post transfer week 2 (PTw2, n=6) group: 14 weeks in SD, then transferred to LD for two weeks; post transfer week 8 (PTw8, n=6) 14 weeks in SD, then transferred to LD for eight weeks. Hamsters were anesthetized and euthanized and ovaries were removed as above.
Total RNA extraction and cDNA synthesis
Ovarian RNA was extracted using Trizol LS reagent (Invitrogen Life Technologies, Carlsbad, CA) according to manufacturer directions. One µg of total RNA (260/280 ratio >1.6) was used for cDNA synthesis using ImProm Reverse Transcription System (Promega, Madison, WI) according to the manufacturers’ directions in a total volume of 20 µl as described (Shahed and Young 2010). cDNA was diluted 1:5 with water (RNAase DNase free) and used for qPCR analysis.
Relative real time PCR
The relative real time PCR was done on Mx3000 thermocycler using Absolute QPCR SYBR green mix (Thermo Fisher Scientific ABgene, Surrey, UK) as described before (Shahed and Young, 2011). Briefly, the qPCR reaction mix contained 1µl cDNA (1:5 dilution of cDNA transcribed using 1µg total RNA) + 1 µl each of forward and reverse primers (80nM concentration) + 6µl SYBRgreen mix + 3 µl water (Promega DNase, RNase, Protease free) to a total volume of 12 µl. PCR cycles consisted of 15 min hold at 95°C (1 cycle), then 40 amplification cycles at appropriate Tm (Table 5), extension (1 min at 72°C) followed by dissociation. Non-template negative controls and standards were included in the same plate as unknown samples. In addition, qPCR products were analyzed on agarose gels to confirm that the correct size (base pairs, Table 4) product was obtained and also to visualize potential secondary and nonspecific amplification. A four point standard curve (efficiency 80 to 100%, correlation coefficient > 0.96) was done by pooling equal aliquots of cDNAs from all samples and diluted fivefold and amplified as above using the specific primers for gene of interest and housekeeping genes (Shahed and Young 2011). The relative amounts of mRNA were calculated using standard curves of each gene of interest and house keeping genes GAPDH and HPRT1 (Tan et al., 2012). Subsequently the ratios of gene of interest to both house keeping genes were calculated. Since ratios between normalizations to both housekeeping genes; therefore for simplicity, only mean ± SEM of the HPRT1-normalized ratios are presented.
Table 5.
Primer Information
| Name | Sequence | Tm | bp | References |
|---|---|---|---|---|
| AMH | F- gca tgg cca act ggt aca ct R-gat gtg tgt gtg agg cct tg |
60 | 153 | Primer sequence provided by Dr. Ned Place |
| Inhibin α | F- ctg ccc tca aca tct cct tc R-ctc atg ctc cct ggt aga gc |
60 | 211 | Gen bank Acc. NM012590 |
| GDF-9 | F-gcg gtc agg cat cgg tat R-aat ggt caa cac gct caa gg |
60 | 100 | Wang and Roy (2006) |
| BMP-15 | F- tat ttc ttg ccc cgt ctt tg R-atc ttg caa agc ttg gtg ct |
56 | 111 | Gen bank Acc. NM009757 |
| HPRT-1 | F- tga tca gtc aac agg gga ca R- ctg gcc gat atc caa cac tt |
55 | 209 | Shahed and Young, 2011 |
Immunohistochemical detection of AMH, Inhibin, BMP-15 and GDF-9 proteins
Paraffin embedded ovary tissue was sectioned (6µ) and deparaffinized in xylene, rehydrated through a graded series of ethanol, washed in phosphate buffer (PBS), and heated in Antigen Unmasking Solution Citra (Vector Laboratories Burlingame, CA) for 8 min at high power in microwave and then cooled in water and washed in PBS (Shahed and Young 2011). Sections were then placed in 0.3% hydrogen peroxide/methanol solution, blocked with species matched normal horse serum/Tween-20 (0.1%) for 40 min, washed twice in PBS incubated with either: anti MIS goat polyclonal (1: 1000; Santa Cruz Biotechnology Inc. Santa Cruz, CA), anti-GDF9 polyclonal antibody (1:50) inhibin-α antibody (1:20–40; Thermo Fisher Scientific Rockford, IL.) or BMP-15 antibody (1:50; Thermo Fisher Scientific Rockford, IL.) for one hour at room temperature followed by overnight at 4°C in humidified chamber. Sections were then processed using a biotinylated pan-specific secondary antibody from the Ready to Use Vectastain Universal Quick Kit and NovaRed Substrate (Vector Laboratories, Burlingame, CA) as per manufacturer’s protocol, counterstained with hematoxylin, dehydrated, and mounted. Negative controls were processed without primary antibody.
Quantification of immunostaining intensity was separately recorded for each protein: preantral follicles (PA), antral follicles (A), corpora lutea (CL), stroma between follicles and CL (containing mostly connective/endothelial tissue, Stroma CT), and stroma containing potentially steroidogenic interstitial cells not incorporated into a defined follicle (Stroma Ste). A staining intensity index was developed following methods described by Vrooman and Young 2010. Briefly, a numerical value of 0–4 was assigned to each structure where: a score of 0 indicated no staining; a score of 1 meant some faint staining in the structures of the subtype being scored, a score of 2 indicated light staining in the structures of the subtype being scored, a score of 3 specified medium-intense staining in the structures of the subtype being scored, and a score of 4 specified intense staining in the structures of the subtype being scored. Because a minimum of three ovarian cross sections per hamster that showed each structure type was required for that individual to be included in the group analysis of that structure type, all structures were not scored for all groups. For example, regressed ovaries of hamsters responding to SD photoperiod lack both antral follicles and corpora lutea, and were therefore not scored for these structures. For all counts, scores for three-six ovarian cross sections (60–100 microns apart at minimum) per animal were averaged, and included in the group mean (n = 4–6 animals per group) used in the ANOVA analysis.
Statistical analysis
Results are presented as mean ± SEM and analyzed by one-way ANOVA with Newman Keuls post hoc tests using Prism 4 software (GraphPad Software, Inc, San Diego, CA). p≤ 0.05 was considered statistically significant.
Acknowledgements
The authors thank Dr. Ned Place at Cornell University for graciously providing the AMH primer sequence and for helpful discussion and mentorship. We also thank Lisa Vrooman, Carling McMichael, Jonae Perez and Dr. Kristy Forsgren for assistance in tissue collection, processing, and sectioning. Finally, we are grateful for the valuable suggestions and comments of two anonymous reviewers, and are appreciative for the kindness of Dr. Julian Wong. Support for this project was provided by the National Institute of General Medical Sciences, NIH SCORE: SC3GM089611 (KAY).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute Of General Medical Sciences or the National Institutes of Health.
Abbreviations
- AAALAC
Association for Assessment and Accreditation of Laboratory Animal Care
- AMH
anti-Müllerian hormone
- BMP-15
bone morphogenic protein 15
- cDNA
copy of deoxyribonucleic acid
- CL
corpus luteum or corpora lutea
- DI
diestrus I
- DII
diestrus II
- E
estrus
- FSH
follicle stimulating hormone
- GDF-9
growth differentiation factor 9
- HPRT
hypoxanthine phosphoribosyal transferase 1
- inh-α
inhibin-α
- LD
long day photoperiod exposure 16 hours of light 8 hours of dark
- LH
luteinizing hormone
- MIS
Müllerian inhibitory substance
- mRNA
messenger ribonucleic acid
- P
proestrus
- PBS
phosphase buffered saline solution
- PTw2
post-transfer from short to long photoperiod for 2 weeks
- PTw8
post-transfer from short to long photoperiod for 8 weeks
- qPCR
real time polymerase chain reaction
- RNA
messenger ribonucleic acid
- SD
short day photoperiod exposure 8 hours of light 16 hours of dark
- TGF-β
transforming growth factor beta
References
- Andersen CY, Schimidt KT, Kristensen SG, Rosendahl M, Byskov AG, Ernst E. Concentration of AMH and inhibin B in relation to follicular diameter in normal human small antral follicles. Human Reproduction. 2010;25:1282–1287. doi: 10.1093/humrep/deq019. [DOI] [PubMed] [Google Scholar]
- Baarends WM, Uilenbroek JT, Kramer P, Hoogerbrugge JW, van Leeuwen EC, Themmen AP, Grootegoed JA. Anti-Müllerian hormone and anti-Müllerian hormone type II receptor messenger ribonucleic acid expression in rat ovaries during post natal development, the estrus cycle and gonadotropin induced follicle growth. Endocrinology. 1995;136:4951–4962. doi: 10.1210/endo.136.11.7588229. [DOI] [PubMed] [Google Scholar]
- Berkowitz AS, Heindel JJ. Inhibin production by Sertoli cells during testicular regression in the golden hamsters. J Androl. 1987;8:272–277. doi: 10.1002/j.1939-4640.1987.tb03321.x. [DOI] [PubMed] [Google Scholar]
- Bernard DJ, Chapman SC, Woodruff TK. Mechanism of inhibin signal transduction. Rec Prog in Hormone Res. 2001;56:417–450. doi: 10.1210/rp.56.1.417. [DOI] [PubMed] [Google Scholar]
- Buchanan KL, Yellon SM. Delayed puberty in the male Djungarian hamster: effect of short photoperiod or melatonin treatment. Neuroendocrinology. 1991;54:96–102. doi: 10.1159/000125857. [DOI] [PubMed] [Google Scholar]
- Culler MD, Merchenthaler I, Heindel JJ, Weisel WC, Negrovilar A. Role of inhibin during long to short photoperiod transition in the hamster. Program for the Annual meeting of the Society for the study of reproduction. 1991 Abstract # 389. [Google Scholar]
- Dodge HH, Kristal MB, Badura LL. Male-induced estrus syncrhonization in female Siberian hamster (Phodopus sungorus) Physiol Behav. 2002;77:227–231. doi: 10.1016/s0031-9384(02)00851-x. [DOI] [PubMed] [Google Scholar]
- Dube JL, Wang P, Elvin J, Lyons KM, Celeste AJ, Matzuk MM. The bone morphogenetic protein 15 gene is X-linked and expressed in oocytes. Mol Endocrinol. 1998;12:1809–1817. doi: 10.1210/mend.12.12.0206. [DOI] [PubMed] [Google Scholar]
- Durlinger AL, Visser JA, Themmen AP. Regulation of ovarian function:the role of anti-Müllerian hormone. Reproduction. 2002;124:601–607. doi: 10.1530/rep.0.1240601. [DOI] [PubMed] [Google Scholar]
- Durlinger AL, Gruijters MJG, Kramer P, Karels B, Ingraham HA, Nachtigal MW, Uilenbroek JTJ, Gorootegoed JA, Themmen APN. Anti-Müllerian hormone inhibits initiation of primordial follicle growth in the mouse ovary. Endocrinology. 2002a;143:1076–1084. doi: 10.1210/endo.143.3.8691. [DOI] [PubMed] [Google Scholar]
- Durlinger AL, Kramer P, Karels B, de Jong FH, Uilenbroek JTJ, Gorootegoed JA, Themmen APN. Anti-Müllerian hormone attenuates the effects of FSH on follicle development in the mouse ovary. Endocrinology. 2001;142:4891–4899. doi: 10.1210/endo.142.11.8486. [DOI] [PubMed] [Google Scholar]
- Durlinger AL, Kramer P, Karels B, de Jong FH, Uilenbroek JTJ, Gorootegoed JA, Themmen APN. Control of primordial follicle recruitment by anti-Müllerian hormone in the mouse ovary. Endocrinology. 1999;140:5789–5796. doi: 10.1210/endo.140.12.7204. [DOI] [PubMed] [Google Scholar]
- El-Fouly MA, Cook B, Nikola M, Nalbandov AV. Role of the ovum in follicular luteinization. Endocrinology. 1970;87:288–293. [PubMed] [Google Scholar]
- Elvin JA, Clark AT, Wang P, Wolfman NM, Matzuk MM. Paracrine action of growth differentiation factor −9 in the mammalian ovary. Mol Endocrinol. 1999;13:1035–1048. doi: 10.1210/mend.13.6.0310. [DOI] [PubMed] [Google Scholar]
- Ericson GF, Shimasaki S. The spatiotemporal expression pattern of bone morphogenetic protein family in rat ovary cell types during the estrus cycle. Repro Biol Endocrinol. 2003;1:9–20. doi: 10.1186/1477-7827-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick SL, Sindoni DM, Shughrue PJ, Lane MV, Merchenthaler IJ, Frail DE. Expression of growth differentiation factor-9 messenger ribonucleic acid in ovarian and nonovarian rodent and human tissues. Endocrinology. 1998;139:2571–2578. doi: 10.1210/endo.139.5.6014. [DOI] [PubMed] [Google Scholar]
- Gilchrist RB, Lane M, Thompson JG. Oocyte-secreted factors: regulators of cumulus cell function and oocyte quality. Hum Reprod Update. 2008;141:59–177. doi: 10.1093/humupd/dmm040. [DOI] [PubMed] [Google Scholar]
- Glass JD. Short photoperiod-induced gonadal regression: effects on the gonadotropin-releasing hormone (GnRH) neuronal system of the white-footed mouse, Peromyscus leucopus. Biology of Reproduction. 1986;35:733–743. doi: 10.1095/biolreprod35.3.733. [DOI] [PubMed] [Google Scholar]
- Gruijters MJG, Visser JA, Durlinger ALL, Themmen APN. Anti-Müllerian hormone and its role in ovarian function. Mol Cell Endocrinol. 2003;211:85–90. doi: 10.1016/j.mce.2003.09.024. [DOI] [PubMed] [Google Scholar]
- Hsueh AJ, Dahl KD, Vaughan J, Tucker E, Rivier J, Bardin CW, Vale W. Hetrodimers and homodimers of inhibin subunits have different paracrine actions in the modulation of lutenizing hormone-stimulated androgen synthesis. PNAS. 1987;84:5082–5086. doi: 10.1073/pnas.84.14.5082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussein TS, Froiland DA, Amato F, Thompson JG, Gilechrist RB. Oocytes prevent cumulus cell apoptosis by maintinaing a morphogeneic paracrine gradient of bone morphogenetic proteins. J Cell Sci. 2005;118:5252–5268. doi: 10.1242/jcs.02644. [DOI] [PubMed] [Google Scholar]
- Jaatinen R, Laitinen MP, Vuojolainen K, Aaltonen J, Louhio H, Heikinheimo K, Lehtonen E, Ritvos O. Localization of growth differentiation factor-9 (GDF9) mRNA and protein in rat ovaries and cDNA cloning of rat GDF9 and its novel homolog GDF9B. Mol Cell Endocrinol. 1999;156:189–193. doi: 10.1016/s0303-7207(99)00100-8. [DOI] [PubMed] [Google Scholar]
- Jayawardana BC, Shimazu T, Nishimoto H, Knaeko E, Tetsuka M, Miyamoto A. Hormonal regulation of expression of growth differentiation factor-9 receptor type I and II in bovine ovarian follicles. Reproduction. 2006;13:545–553. doi: 10.1530/rep.1.00885. [DOI] [PubMed] [Google Scholar]
- Kabithe EW, Place NJ. Photoperiod-dependent modulation of anti-Müllerian hormone in female Siberian hamsters, Phodopus sungorus . Reproduction. 2008;135:335–342. doi: 10.1530/REP-07-0423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny HA, Bernard DJ, Horton TH, Woodruff TK. Photoperiod-dependent regulation of inhibins in Siberian hamsters: I. Ovarian inhibin production and secretion. J Endocrinol. 2002;174:71–83. doi: 10.1677/joe.0.1740071. [DOI] [PubMed] [Google Scholar]
- Kenny HA, Bernard DJ, Horton TH, Woodruff TK. Photoperiod-dependent regulation of inhibin in Siberian hamsters: II. Regulation of inhibin production and secretion by pregnant mare serum gonadotropin. J Endocrinol. 2002;174:85–94. doi: 10.1677/joe.0.1740085. [DOI] [PubMed] [Google Scholar]
- Kenny HA, Woodruff TA. Follicle size contributes to distinct secretion patterns of inhibin isoforms during rat estrus cycle. Endocrinology. 2006;147:51–60. doi: 10.1210/en.2005-0242. [DOI] [PubMed] [Google Scholar]
- Kevenaar ME, Meersahib MF, Kramer P, Van de Lang-Born BM, De Jong FH, Groome MP, Themmen AP. Anit-Müllerian hormone levels reflect the size of reflect the size of the primordial follicle pool in mice. Endocrinol. 2006;147:3228–3234. doi: 10.1210/en.2005-1588. [DOI] [PubMed] [Google Scholar]
- Knight PG, Glister C. TGF-β superfamily and ovarian follicle development. Reproduction. 2006;132:191–206. doi: 10.1530/rep.1.01074. [DOI] [PubMed] [Google Scholar]
- Laitinen R, Laitinen MP, Vuojolainin K, Aalotnen J, Louhio H, Heikinheimo K, Lehtonen E, Ritvos O. Localization of growth differentiation factor-9(GDF-9) mRNA and protein in rat ovaries and cDNA cloning of rat GDF-9 and its novel homolog GDF-9B. Mol Cellular Endocrinol. 1999;156:189–193. doi: 10.1016/s0303-7207(99)00100-8. [DOI] [PubMed] [Google Scholar]
- McGee EA, Hsueh AJ. Initial and cyclic recruitment of ovarian follicles. Endocrine Reviews. 2000;21:200–214. doi: 10.1210/edrv.21.2.0394. [DOI] [PubMed] [Google Scholar]
- McGrath SA, Esquela AF, Lee S-J. Oocyte-specific expression of growth/differentiation factor-9. Mol Endocrinol. 1995;9:131–136. doi: 10.1210/mend.9.1.7760846. [DOI] [PubMed] [Google Scholar]
- Meunier H, Cajander SB, Roberts VJ, Rivier C, Sawchenko PE, Hsueh AJ, Vale W. Rapid changes in the expression of inhibin alpha-, beta A-, and beta B-subunits in ovarian cell types during the rat estrous cycle. Mol Endocrinol. 1988;2:1352–1363. doi: 10.1210/mend-2-12-1352. [DOI] [PubMed] [Google Scholar]
- Moffatt-Blue CS, Sury JJ, Young KA. Short photoperiod-induced ovarian regression is mediated by apoptosis in Siberian hamsters (Phodopus sungorus) Reproduction. 2006;131:771–782. doi: 10.1530/rep.1.00870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monti M, Redi C. Oogenesis specific genes (Nobox, Oct4, Bmp15, Gdf9, Oogenesin1 and Oogenesin2) are differentially expressed during natural and gonadotropin-induced mouse follicular development. Mol Reprod Dev. 2009;76:994–1003. doi: 10.1002/mrd.21059. [DOI] [PubMed] [Google Scholar]
- Mottershead DG, Ritter LJ, Gilchrist RB. Signalling pathways mediating specific synergistic interactions between GDF-9 and BMP-15. Mol Hum Repro. 2012;18:121–128. doi: 10.1093/molehr/gar056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata T, Takizawa T, Funaba M, Ujimura H, Murata E, Takahashi M, Torii K. Quatitative RT-PCR for inhibin/Activin subunits: measurement of rat hypothalamic and ovarian inhibin/activin subunit mRNAs during the estrus cycle. Endocrine J. 1997;44:35–42. doi: 10.1507/endocrj.44.35. [DOI] [PubMed] [Google Scholar]
- Myers M, Pangas SA. Regulatory roles of transforming growth factor beta superfamily in folliculogenesis. Wiley Interdiscip Rev Syst Biol. 2010;2:117–125. doi: 10.1002/wsbm.21. [DOI] [PubMed] [Google Scholar]
- Nilsson EE, Schindler R, Savenkova MI, Skinner MK. Inhibitory actions of anti-Müllerian hormone (AMH) on ovarian primordial follicle assembly. PloS one. 2011;6:e20087. doi: 10.1371/journal.pone.0020087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orisaka M, Orisaka S, Jiang JY, Wang Y, Kotsuji F, Tsan BK. Growth differentiation factor 9 is antiapoptotic during follicular development from preantral to early antral stage. Mol Endocrinol. 2006;20:2456–2468. doi: 10.1210/me.2005-0357. [DOI] [PubMed] [Google Scholar]
- Otsuka F, McTavish K, Shimasaki S. Integral role of GDF-9 and BMP-15 in ovarian function. Mol Reprod Dev. 2011;78:9–21. doi: 10.1002/mrd.21265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Place NJ, Cruickshank J. Graded response to short photoperiod during development and early adulthood in Siberian hamsters and the effects on reproduction as females age. Horm. Behav. 2009;55:390–397. doi: 10.1016/j.yhbeh.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao JN, Chandrashekar V, Borg KE, Bartke A. Effects of photoperiod on testicular inhibin-alpha and androgen binding protein mRNA expression during postnatal development in Siberian hamsters, Phodopus sungorus. Life Sci. 1995;57:1761–1770. doi: 10.1016/0024-3205(95)02154-b. [DOI] [PubMed] [Google Scholar]
- Sadeu JC, Adriaenssens T, Smitz J. Expression of growth and differentiation factor 9, bonemorphogenetic protein-15 and anti-Müllerian hormone in cultured mouse primary follicles. 2008;136:195–203. doi: 10.1530/REP-08-0065. [DOI] [PubMed] [Google Scholar]
- Salverson TJ, McMichael GE, Sury JJ, Shahed A, Young KA. Differential expression of matrix metalloproteinases during stimulated ovarian recrudescence in Siberian hamsters (Phodopus sungorus) Gen Comp Endocrinol. 2008;155:749–761. doi: 10.1016/j.ygcen.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlatt P, Niklowitz K, Hoffmann E, Nieschlag Influence of short photoperiods on reproductive organs and estrous cycles of normal and pinealectomized female Djungarian hamsters, Phodopus sungorus. Biol Reprod. 1993;49:243–250. doi: 10.1095/biolreprod49.2.243. [DOI] [PubMed] [Google Scholar]
- Shahed A, Young KA. Intraovarian expression of GnRH-1 and gonadotropin mRNA and protein levels in Siberian hamster during the estrus cycle and photoperiod induced regression/recrudescence. Gen Comp Endocrinol. 2011;170:356–364. doi: 10.1016/j.ygcen.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva JR, van den Hurk R, van Tol HT, Roelen BA, Figureiredo JR. Expression of growth differentiation factor 9 (GDF-9), Bone morphogenetic protein 15(BMP-15), and BMP-15 receptors in the ovaries of goats. Mol Repro Dev. 2005;70:11–19. doi: 10.1002/mrd.20127. [DOI] [PubMed] [Google Scholar]
- Su YQ, Sugiura K, Eppig JJ. Mouse oocyte control of granulosa cell development and function: paracrine regulation of cumulus cell metabolism. Semin Reprod Med. 2009:2732–2742. doi: 10.1055/s-0028-1108008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun RZ, Lei L, Cheng L, Jin ZF, Zu SJ, Shan ZY, Wang SD, Zhang JK, Liu ZH. Expression of GDF-9, BMP-15 and their receptors in mammaliean ovary follicles. J Mol Hist. 2010;41:325–332. doi: 10.1007/s10735-010-9294-2. [DOI] [PubMed] [Google Scholar]
- Tan SC, Carr CA, Yeoh KK, Schofield CJ, Davies KE, Calrke K. Identification of valid housekeeping genes for quantitative RT-PCR analysis of cardiosphere -derived cells preconditioned under hypoxia or with proplyl-4- hydroxylase inhibitors. Mol Biol Rep. 2012;39:4857–4867. doi: 10.1007/s11033-011-1281-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trombly D, Woodruff TK, Mayo K. Roles of transforming growth factor beta superfamily proteins in early folliculogenesis. Semin Repro Med. 2009;27:14–23. doi: 10.1055/s-0028-1108006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Hurk R, Diijkstra G, De Jong F. Enhanced serum oestrogen levels and highly steroidogenic, lutenized atretic follicles in the ovaries of the Djungarian hamsters (Phodopus sungorus) kept under short photoperiod from birth. Eur J Endocrinol. 2002;147:7011–7710. doi: 10.1530/eje.0.1470701. [DOI] [PubMed] [Google Scholar]
- Visser AA, Durlinger ALL, Peters IJJ, Edwin R, Heuvel VD, Rose UM, Kramer P, de Jong F, Themmen APN. Increased oocyte degeneration and follicular the estrous cycle in anti-Müllerian hormone null mice. Endocrinology. 2007;148:2301–2308. doi: 10.1210/en.2006-1265. [DOI] [PubMed] [Google Scholar]
- Vrooman LA, Young KA. Ovarian matrix metalloproteinases are differentially regulated during the estrous cycle but not during short photoperiod induced regression in Siberian hamsters (Phodopus sungorus) Reprod Biol Endocrinol. 2010;8:79. doi: 10.1186/1477-7827-8-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Roy SK. Growth differentiation factor 9 promote primordial follicle formation in the hamster: modulation by follicle stimulating hormone. Biol Reprod. 2004;70:577–585. doi: 10.1095/biolreprod.103.023234. [DOI] [PubMed] [Google Scholar]
- Wang C, Roy SK. Expression of growth and differentiation factor 9 in the oocyltes is essential for the development of primordial follicles in the hamster ovary. Endocrinol. 2006;147:1725–1734. doi: 10.1210/en.2005-1208. [DOI] [PubMed] [Google Scholar]
- Wanzu J, Chandana BH, Midori Y, Koji YA, Erina S, Shi Z, Longquan R, Gen W, Nigel PG, Kazuyoshi T. Inhibin B regulating follicle-stimulating hormone secretion during testicular recrudescence in the male golden hamster. J Androl. 2002;23:1725–1734. [PubMed] [Google Scholar]
- Weenen C, Laven JS, Anne RM, Von Bergh AR, Cranfield M, Groome NP, Visser JA, Kramer P, Fauser BC, Themmen APN. Anti-Müllerian hormone expression pattern in human ovary: potential implications for initial and cyclic recruitment. Mol Hum Repro. 2004;10:77–83. doi: 10.1093/molehr/gah015. [DOI] [PubMed] [Google Scholar]
- Woodruff TK, Besecke LM, Groome N, Draper LB, Schwartz NB, Weiss J. Inhibin A and Inhibin B are inversely correlated to follicle stimulating hormone, yet are discordant during the follicular phase of the rat estrus cycle, and inhibin A is expressed in a sexually dimorphic manner. Endocrinology. 1996;137:5463–5467. doi: 10.1210/endo.137.12.8940372. [DOI] [PubMed] [Google Scholar]
- Yan C, Wang P, DeMayo FJ, Elvin JA, Carino C, Prasad SV, Skinner SS, Dunbar BS, Dube JL, Celeste AJ, Matzuk MM. Synergistic roles of bonemorphogenetic protein 15 and growth differentiation factor 9 in ovarian function. Mol Endocrinol. 2001;15:854–866. doi: 10.1210/mend.15.6.0662. [DOI] [PubMed] [Google Scholar]
- Yellon SM, Goldman BD. Influence of short days on diurnal patterns of serum gonadotrophins and prolactin concentrations in the male Djungarian hamster, Phodopus sungorus. Journal of Reproduction and Fertility. 1987;80:167–174. doi: 10.1530/jrf.0.0800167. [DOI] [PubMed] [Google Scholar]
- Zysling DA, Park S, McMillan EL, Place NJ. Photoperiod-gonadotropin mismatches induced by treatment with acycline or FSH in Siberian hamsters: impacts on ovarian structure and function. Reproduction. 2012;5:603–616. doi: 10.1530/REP-12-0155. [DOI] [PubMed] [Google Scholar]


