Abstract
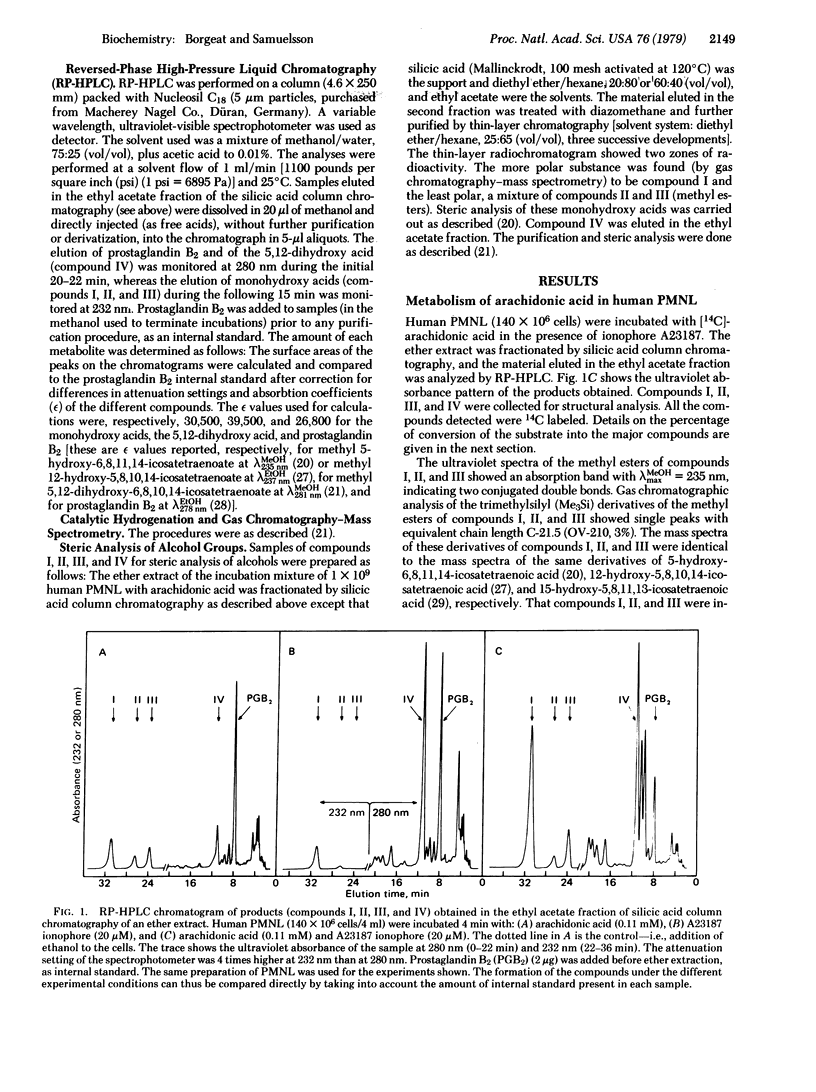
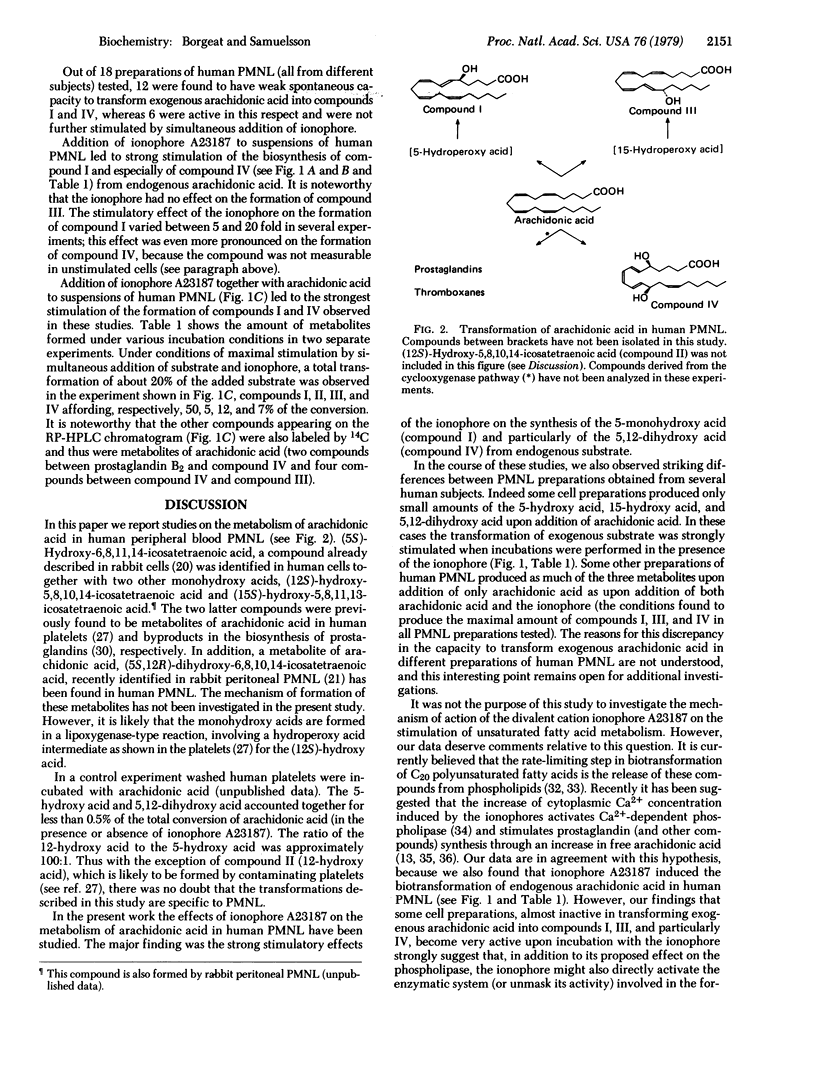
Addition of arachidonic acid and the divalent cation ionophore A23187 to a suspension of human peripheral blood polymorphonuclear leukocytes led to the formation of (5S)-hydroxy-6,8,11,14-icosatetraenoic acid, (15S)-hydroxy-5,8,11,13-icosatetraenoic acid, and (5S,12R)-dihydroxy-6,8,10,14-icosatetraenoic acid. A method based on high-pressure liquid chromatography has been developed for assay of these metabolites. The addition of arachidonic acid to human polymorphonuclear leukocytes always resulted in formation of the isomeric monohydroxy acids. However, cells prepared from blood of different subjects were found to vary with respect to formation of the 5,12-dihydroxy acid. Addition of the ionophore alone strongly stimulated the formation of the 5-monohydroxy acid and more specifically the 5,12-dihydroxy acid from endogenous arachidonic acid. In all experiments performed the formation of the 5-hydroxy acid and the 5,12-dihydroxy acid was maximally stimulated when both arachidonic acid and the ionophore were added to the incubation mixture. Under these conditions, stimulation of 40-fold or more of the formation of both compounds was observed. The data demonstrate that, in addition to causing release of endogenous substrate, the ionophore also activated the enzymatic system involved in the further transformations of arachidonic acid. This finding raises the possibility that this pathway of arachidonic acid metabolism is involved in the biological response (e.g., release of lysosomal enzymes, the slow reacting substance of anaphylaxis, and chemotactic factors) of leukocytes to A23187 and other stimuli.
Keywords: monohydroxy acids, dihydroxy acid, prostaglandins, human neutrophils
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asano T., Hidaka H. Purification of guanylate cyclase from human platelets and effect of arachidonic acid peroxide. Biochem Biophys Res Commun. 1977 Oct 10;78(3):910–918. doi: 10.1016/0006-291x(77)90509-5. [DOI] [PubMed] [Google Scholar]
- BERGSTROEM S., RYHAGE R., SAMUELSSON B., SJOEVALL J. PROSTAGLANDINS AND RELATED FACTORS. 15. THE STRUCTURES OF PROSTAGLANDIN E1, F1-ALPHA, AND F1-BETA. J Biol Chem. 1963 Nov;238:3555–3564. [PubMed] [Google Scholar]
- BOYSE E. A., OLD L. J., CHOUROULINKOV I. CYTOTOXIC TEST FOR DEMONSTRATION OF MOUSE ANTIBODY. Methods Med Res. 1964;10:39–47. [PubMed] [Google Scholar]
- Borgeat P., Hamberg M., Samuelsson B. Transformation of arachidonic acid and homo-gamma-linolenic acid by rabbit polymorphonuclear leukocytes. Monohydroxy acids from novel lipoxygenases. J Biol Chem. 1976 Dec 25;251(24):7816–7820. [PubMed] [Google Scholar]
- Boyle W. An extension of the 51Cr-release assay for the estimation of mouse cytotoxins. Transplantation. 1968 Sep;6(6):761–764. doi: 10.1097/00007890-196809000-00002. [DOI] [PubMed] [Google Scholar]
- Bøyum A. Isolation of lymphocytes, granulocytes and macrophages. Scand J Immunol. 1976 Jun;Suppl 5:9–15. [PubMed] [Google Scholar]
- Conroy M. C., Orange R. P., Lichtenstein L. M. Release of slow reacting substance of anaphylaxis (SRS-A) from human leukocytes by the calcium ionophore A23187. J Immunol. 1976 Jun;116(6):1677–1681. [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. Plaque formation and isolation of pure lines with poliomyelitis viruses. J Exp Med. 1954 Feb;99(2):167–182. doi: 10.1084/jem.99.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derksen A., Cohen P. Patterns of fatty acid release from endogenous substrates by human platelet homogenates and membranes. J Biol Chem. 1975 Dec 25;250(24):9342–9347. [PubMed] [Google Scholar]
- Falardeau P., Hamberg M., Samuelsson B. Metabolism of 8,11,14-eicosatrienoic acid in human platelets. Biochim Biophys Acta. 1976 Aug 23;441(2):193–200. doi: 10.1016/0005-2760(76)90162-4. [DOI] [PubMed] [Google Scholar]
- Gallin J. I., Sandler J. A., Clyman R. I., Manganiello V. C., Vaughan M. Agents that increase cyclic AMP inhibit accumulation of cGMP and depress human monocyte locomotion. J Immunol. 1978 Feb;120(2):492–496. [PubMed] [Google Scholar]
- Goetzl E. J., Gorman R. R. Chemotactic and chemokinetic stimulation of human eosinophil and neutrophil polymorphonuclear leukocytes by 12-L-hydroxy-5,8,10-heptadecatrienoic acid (HHT). J Immunol. 1978 Feb;120(2):526–531. [PubMed] [Google Scholar]
- Goetzl E. J., Woods J. M., Gorman R. R. Stimulation of human eosinophil and neutrophil polymorphonuclear leukocyte chemotaxis and random migration by 12-L-hydroxy-5,8,10,14-eicosatetraenoic acid. J Clin Invest. 1977 Jan;59(1):179–183. doi: 10.1172/JCI108617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gzarnetzki B. M., Konig W., Lichtenstein L. M. Release of eosinophil chemotactic factor from human polymorphonuclear neutrophils by calcium ionophore A23187 and phagocytosis. Nature. 1975 Dec 25;258(5537):725–726. doi: 10.1038/258725a0. [DOI] [PubMed] [Google Scholar]
- Hamberg M., Samuelsson B. Prostaglandin endoperoxides. Novel transformations of arachidonic acid in human platelets. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3400–3404. doi: 10.1073/pnas.71.9.3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamberg M., Samuelsson B. Prostaglandin endoperoxides. VII. Novel transformations of arachidonic acid in guinea pig lung. Biochem Biophys Res Commun. 1974 Dec 11;61(3):942–949. doi: 10.1016/0006-291x(74)90246-0. [DOI] [PubMed] [Google Scholar]
- Hamberg M. Steric analysis of hydroperoxides formed by lipoxygenase oxygenation of linoleic acid. Anal Biochem. 1971 Oct;43(2):515–526. doi: 10.1016/0003-2697(71)90282-x. [DOI] [PubMed] [Google Scholar]
- Hidaka H., Asano T. Stimulation of human platelet guanylate cyclase by unsaturated fatty acid peroxides. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3657–3661. doi: 10.1073/pnas.74.9.3657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill H. R., Estensen R. D., Quie P. G., Hogan N. A., Goldberg N. D. Modulation of human neutrophil chemotactic responses by cyclic 3',5'-guanosine monophosphate and cyclic 3',5'-adenosine monophosphate. Metabolism. 1975 Mar;24(3):447–456. doi: 10.1016/0026-0495(75)90124-9. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J., George W. J. Hormonal control of lysosomal enzyme release from human neutrophils: elevation of cyclic nucleotide levels by autonomic neurohormones. Proc Natl Acad Sci U S A. 1974 May;71(5):2027–2031. doi: 10.1073/pnas.71.5.2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp H. R., Oelz O., Roberts L. J., Sweetman B. J., Oates J. A., Reed P. W. Ionophores stimulate prostaglandin and thromboxane biosynthesis. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4251–4255. doi: 10.1073/pnas.74.10.4251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lands W. E., Samuelsson B. Phospholipid precursors of prostaglandins. Biochim Biophys Acta. 1968 Oct 22;164(2):426–429. doi: 10.1016/0005-2760(68)90168-9. [DOI] [PubMed] [Google Scholar]
- Lewis G. P. Prostaglandins in inflammation. J Reticuloendothel Soc. 1977 Oct;22(4):389–402. [PubMed] [Google Scholar]
- Oelz O., Knapp H. R., Roberts L. J., Oelz R., Sweetman B. J., Oates J. A., Reed P. W. Calcium-dependent stimulation of thromboxane and prostaglandin biosynthesis by ionophores. Adv Prostaglandin Thromboxane Res. 1978;3:147–158. [PubMed] [Google Scholar]
- Pickett W. C., Jesse R. L., Cohen P. Initiation of phospholipase A2 activity in human platelets by the calcium ion ionophore A23187. Biochim Biophys Acta. 1976 Jan 18;486(1):209–213. doi: 10.1016/0005-2760(77)90086-8. [DOI] [PubMed] [Google Scholar]
- Romeo D., Zabucchi G., Miani N., Rossi F. Ion movement across leukocyte plasma membrane and excitation of their metabolism. Nature. 1975 Feb 13;253(5492):542–544. doi: 10.1038/253542a0. [DOI] [PubMed] [Google Scholar]
- Sandler J. A., Gallin J. I., Vaughan M. Effects of serotonin, carbamylcholine, and ascorbic acid on leukocyte cyclic GMP and chemotaxis. J Cell Biol. 1975 Nov;67(2PT1):480–484. doi: 10.1083/jcb.67.2.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schell-Frederick E. Stimulation of the oxidative metabolism of polymorphonuclear leucocytes by the calcium ionophore A23187. FEBS Lett. 1974 Nov 1;48(1):37–40. doi: 10.1016/0014-5793(74)81056-2. [DOI] [PubMed] [Google Scholar]
- Smith R. J., Ignarro L. J. Bioregulation of lysosomal enzyme secretion from human neutrophils: roles of guanosine 3':5'-monophosphate and calcium in stimulus-secretion coupling. Proc Natl Acad Sci U S A. 1975 Jan;72(1):108–112. doi: 10.1073/pnas.72.1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner S. R., Tainer J. A., Lynn W. S. Biogenesis of chemotactic molecules by the arachidonate lipoxygenase system of platelets. Nature. 1975 Oct 23;257(5528):680–681. doi: 10.1038/257680a0. [DOI] [PubMed] [Google Scholar]
- Vonkeman H., van Dorp D. A. The action of prostaglandin synthetase on 2-arachidonyl-lecithin. Biochim Biophys Acta. 1968 Oct 22;164(2):430–432. doi: 10.1016/0005-2760(68)90169-0. [DOI] [PubMed] [Google Scholar]
- Weidemann M. J., Peskar B. A., Wrogemann K., Rietschel E. T., Staudinger H., Fischer H. Prostaglandin and thromboxane synthesis in a pure macrophage population and the inhibition, by E-type prostaglandins, of chemiluminescence. FEBS Lett. 1978 May 1;89(1):136–140. doi: 10.1016/0014-5793(78)80539-0. [DOI] [PubMed] [Google Scholar]
- Wentzell B., Epand R. M. Stimulation of the release of prostaglandins from polymorphonuclear leukocytes by the calcium inophore A23187. FEBS Lett. 1978 Feb 15;86(2):255–258. doi: 10.1016/0014-5793(78)80574-2. [DOI] [PubMed] [Google Scholar]
- Zabucchi G., Soranzo M. R., Rossi F. Exocytosis in human polymorphonuclear leukocytes induced by A 23187 and calcium. FEBS Lett. 1975 Jun 1;54(1):44–48. doi: 10.1016/0014-5793(75)81064-7. [DOI] [PubMed] [Google Scholar]
- Zurier R. B., Weissmann G., Hoffstein S., Kammerman S., Tai H. H. Mechanisms of lysosomal enzyme release from human leukocytes. II. Effects of cAMP and cGMP, autonomic agonists, and agents which affect microtubule function. J Clin Invest. 1974 Jan;53(1):297–309. doi: 10.1172/JCI107550. [DOI] [PMC free article] [PubMed] [Google Scholar]


