Abstract
Background
Polymorphism in the IL28B gene region, encoding interferon-lambda(λ)-3, is strongly predictive of response to antiviral treatment in the non-transplant setting. We sought to determine the prevalence and impact on clinical outcomes of donor and recipient IL28B genotypes among liver transplant recipients.
Methods
Cohort study including 189 consecutive hepatitis C virus (HCV) patients who underwent liver transplantation between 1-1-1995 and 1-1-2005 in the Mayo Clinic, Rochester. Genotyping of the polymorphism rs12979860 was performed on DNA collected from all donors and recipients in the cohort. 65 patients received IFN-based antiviral therapy.
Results
The CC IL28B variant was less common in the CHC recipients than in non-HCV donor livers (33% vs 47%, P=0.03). IL28B recipient genotype was significantly predictive of fibrosis stage, with TT genotype being associated with more rapid fibrosis (Pearson Chi-square p=0.024 for the comparison G vs A). Donor and recipient IL28B genotype were independently associated with SVR (P<0.005). The presence of IL28B CC variant in either the recipient (R) or donor (D) liver was associated with increased rate of SVR (D-non-CC / R-non-CC = 3/19 (16%) vs D-CC / R-non-CC=11/22 (50%) vs D-non-CC / R-CC=5/12 (42 %) vs R-CC / D-CC=6/7 (86%), P=0.0095). IL28B genotype was not significantly associated with survival (overall / liver related).
Conclusion
Recipient IL28B genotype is associated with more severe histological recurrence of HCV. Recipient and donor liver IL28B genotype are strongly and independently associated with IFN-based treatment response in patients post-OLT. The data suggest that CC donor livers might be preferentially allocated to patients with HCV infection.
Introduction
Chronic hepatitis C virus (HCV) infection is the most common indication for liver transplantation in the Western world. Recurrence of HCV infection is the most common cause of death and graft loss following liver transplantation 1. Although treatment with interferon (IFN)/ peginterferon (pegIFN) and ribavirin (RBV) is feasible in the post-transplant setting, sustained virological response (SVR) rates are lower than in non-transplant patients 2 and many patients do not tolerate therapy.
Genetic variation in the region of the IL28B gene on chromosome 19, coding for interferon lambda (λ)-3), has recently been demonstrated to be strongly associated with SVR in patients with genotype 1 chronic HCV infection who are treated with pegIFN plus RBV in the non-transplant setting 3-5. IL28B polymorphism has also been associated with spontaneous HCV clearance 6, 7. To date, the mechanism underlying the association between IL28B variants and natural / treatment-induced clearance remains unclear. The role and relevance of IL28B polymorphism (recipient / donor) to HCV recurrence post-transplantation, and the HCV treatment outcome post-transplantation have not previously been investigated.
We have therefore performed a retrospective analysis of a well-characterized HCV transplant cohort in which both recipient and donor liver DNA was tested for IL28B genotype 8. We tested for an association between recipient / donor IL28B variants and treatment outcome, as well as natural history post-transplantation, including time to HCV recurrence and patient survival.
Methods
Patients
Consecutive chronic hepatitis C patients who underwent orthotopic liver transplantation (OLT) between 1-1-1995 and 1-1-2005 in the Mayo Clinic in Rochester, Minnesota were studied. This allowed up to a potential of >/= 5 years of follow up in all patients. DNA was collected prospectively from all donors and recipients. Data were obtained according to a standard protocol. A subset of the patients was treated with IFN-based regimens. The study protocol was approved by the Institutional Review Board of the Mayo Clinic and was carried out in accordance with institutional guidelines.
Laboratory testing
Virological data (genotype, viral load), biochemical and hematological data were measured in the certified Mayo Clinic laboratories. A sensitive qualitative assay was used to detect serum HCV RNA, COBAS Amplicor HCV Test, v. 2.0 assay (Roche Molecular Systems), which has a sensitivity of 100 IU/mL. Genotypes were assigned using nucleotide primers specific for a 401-bp target sequence within the NS5 region with sequence information compared with published HCV type reference sequences using the FASTA algorithm (Wisconsin Genetics Computer Group, Madison, WI).
DNA extraction and IL28B genotyping
DNA was extracted from stored paraffin-fixed liver tissue blocks using the QIAamp DNA Mini Kit (QIagen, Valencia, CA, USA) assay. Tissue was used as whole blood was not available. Donor and recipient DNA was tested for the polymorphism rs12979860 using the ABI TaqMan allelic discrimination kit and the ABI7900HT Sequence Detection System (Applied Biosystems, Carlsbad, CA, USA). The possible genotypes for this bi-allelic polymorphism are: CC, CT and TT, where the CC variant has previously been associated with good response to pegIFN plus RBV therapy in patients infected with genotype 1 HCV 3, 9.
Clinical endpoints
Hepatitis C Recurrence and Allograft Histology: Recurrent hepatitis C was defined histologically. Liver biopsies were performed routinely at 1, 3 and 5 years post-OLT, and also when clinically indicated. Hepatitis C recurrence was defined as the presence of HCV RNA in the serum, as well as allograft histology showing lymphocytic infiltrates suggestive of recurrent hepatitis C infection in the absence of other Banff criteria for acute cellular rejection. Biopsies were read by experienced hepatopathologists who used a standard system for biopsy interpretation. Sustained virological response (SVR): was defined as undetectable serum HCV ribonucleic acid (RNA) at 24 weeks after the end-of-treatment. Follow-up: Re-transplantation and mortality were recorded.
Statistics
Baseline characteristics were compared using Mann Whitney / Kruskal Wallis Tests or χ2 tests for continuous and categorical variables, respectively. Univariate Cox regression analyses and backward and forward stepwise analyses were applied to build a multivariate proportional hazards model assessing risk factors for recurrent HCV infection, and for 5 year graft survival. The following factors were considered: IL28B genotype, gender, age, donor age, diabetes mellitus, BMI corrected for the amount of ascites removed during the transplant procedure, MELD-score, HCV genotype, platelet count, prothrombin time, AST, ALT, bilirubin, gamma glutamyl transferase, creatinine, iothalamate clearance, albumin, sodium, glucose, cholesterol, triglycerides, HCV RNA, warm ischemia, cold ischemia, HCC as an indication for transplantation, alcohol use as a contributing factor to liver disease, race ± time to recurrent hepatitis C, and antiviral therapy, in the analysis of graft survival. Univariate and multivariate logistic regression analyses were performed to assess which factors at the time of recurrence were predictive of SVR after peginterferon treatment. Factors that were statistically significant in univariate analysis with a p-value <0.10 were entered in the multivariate Cox-regression model. Several models were fit, the final model including the covariates with the best fit to the data, according to Chi-square test. All statistical analyses were performed in SAS version 9.1 (SAS institute, Cary, NC, USA).
Results
Study population
220 consecutive chronic hepatitis C patients underwent OLT between 1-1-1995 and 1-1-2005. Only patients alive and not requiring retransplantation in the first 90 postoperative days were included in this analysis. Donor and recipient liver tissue was available for IL28B genotyping in 189 patients. Genotype at the polymorphic site rs12979860 on chromosome 19 was suitable for analysis in 171 recipients (90%) and in 172 donors (91%). In 155 patients both donor and recipient IL28B genotype were successfully characterized. Table 1 shows pre-OLT characteristics.
Table 1.
Baseline patient characteristics before transplantation, according to recipient IL28B genotype
| CC (n=56*) | CT (n=87*) | TT (n=28) | P-value* | |
|---|---|---|---|---|
| Donor age, years | 46 (26-56) | 44 (28-60) | 43 (25-55) | 0.84 |
| Age, years | 51 (46-55) | 48 (44-55) | 50 (46-55) | 0.44 |
| Male gender (%) | 36 (64) | 61 (70) | 19 (68) | 0.77 |
| HCV Genotype (%) | 0.034 | |||
| genotype 1 | 24 (65) | 44 (73) | 21 (91) | |
| genotype 2 | 5 (14) | 8 (13) | 2 (9) | |
| genotype 3 | 8 (22) | 4 (7) | 0 (0) | |
| genotype 4 | 0 (0) | 4 (7) | 0 (0) | |
| MELD¶ | 17 (13-22) | 17 (13-21) | 15 (12-19) | 0.70 |
| Prothrombin time, sec | 13 (11-15) | 14 (12-16) | 13 (11-16) | 0.82 |
| Bilirubin, mg/dL | 2.2 (1.4-4.0) | 2.6 (1.,6-5.1) | 3.2 (1.7-4.1) | 0.46 |
| Creatinine, mg/dL | 1.0 (0.9-1.2) | 1.0 (0.9-1.4) | 1.1 (0.9-1.3) | 0.89 |
| Albumin, g/dL | 3.1 (2.8-3.6) | 3.1 (2.7-3.4) | 2.9 (2.5-3.2) | 0.048 |
| Iothalamate clearance, mL/min | 105 (84-117) | 89 (62-107) | 92 (64-119) | 0.040 |
Continuous variables are expressed as medians (inter quartile range).
MELD = Model for End-Stage Liver Disease
During first month after transplantation
Kruskall Wallis/Chi square
During a median follow-up of 4.6 years (inter quartile range (IQR) 2.4-6.9) 148 patients (80%) had evidence of recurrent hepatitis C, the median time from OLT to diagnosis of recurrence being 1.0 year (IQR 0.3-2.2). Twenty-one patients were diagnosed with recurrent hepatitis C after protocol biopsy at 1 year after OLT, 9 after protocol biopsy at 3 years after OLT and 1 patient after protocol biopsy at 5 years after OLT. The remaining patients were not diagnosed using protocol liver biopsies but by biopsies taken because it was deemed necessary by the treating physician, usually because of a rise in transaminase levels.
IL28B genotype distribution
DNA from 171 liver transplant recipients and 172 donor livers was successfully typed for the polymorphism rs12979860 (Table 2). Both genotype frequencies were in Hardy-Weinberg equilibrium. The CC IL28B variant was significantly less common amongst the transplant recipients, chronically infected with HCV, than the non-HCV donor livers (33% vs 47%, P = 0.03). 155 patients could be defined according to donor : recipient IL28B genotype pairs (CC vs non-CC). The respective frequencies were: donor non-CC : recipient non-CC = 34%, donor CC : recipient non-CC = 32%, donor non-CC : recipient CC = 19%, donor CC : recipient CC = 15% (Table 2).
Table 2.
IL28B genotype frequency
| (A) Overall IL28B genotype frequency | |||
|---|---|---|---|
| IL28B genotype frequency | Overall | Treated | Not treated |
|
| |||
| Recipient* | (n=171) | (n=67) | (n=104) |
| CC | 56 (33%) | 22 (33%) | 34 (33%) |
| CT | 87 (51%) | 33 (49%) | 54 (52%) |
| TT | 28 (16%) | 12 (18%) | 16 (15%) |
| Undetermined | 18 | 10 | 8 |
| HWE**, Ψ2 P-value | 0.55 | ||
| Donor* | (n=172) | (n=74) | (n=98) |
| CC | 80 (47%) | 33 (45%) | 47 (48%) |
| CT | 72 (42%) | 36 (49%) | 36 (37%) |
| TT | 20 (12%) | 5 (7%) | 15 (15%) |
| Undetermined | 17 | 3 | 14 |
| HWE**, Ψ2 P-value | 0.54 | ||
| Donor vs recipient, Ψ2 P-value | 0.0305 | ||
| (B), Paired recipient:donor IL28B genotype frequency | |||
|---|---|---|---|
| IL28B genotype frequency | Overall | Treated | Not treated |
|
| |||
| (n=155) | (n=65) | (n=90) | |
| Donor non-CC : Recipient non-CC | 52 (34%) | 19 (29%) | 33 (37%) |
| Donor CC : Recipient non-CC | 50 (32%) | 24 (37%) | 26 (29%) |
| Donor non-CC : Recipient CC | 29 (19%) | 15 (23%) | 14 (16%) |
| Donor CC : Recipient CC | 24 (15%) | 7 (11%) | 17 (19%) |
IL28B genotype was not determined from 18 recipients and 17 donor livers
HWE = Hardy-Weinberg equilibrium
Post-transplant Outcomes
All patients had virological recurrence of HCV infection following liver transplantation. 110/171 (64%) of recipients in whom the IL28B SNP was successfully genotyped were diagnosed with recurrent hepatitis C by the fifth postoperative year. Time to recurrence was delayed in recipients with the CC IL28B genotype compared to CT and TT genotypes (5-year recurrence: 78% vs 87% vs 100% respectively, P = 0.0173).
Multivariate Cox regression analysis showed that the recipient IL28B C allele was an independent predictor of delayed recurrence of hepatitis C at 2 years: HR 0.619, 95% CI 0.434 - 0.883, P=0.0081 (Table 3). Pre-transplant MELD score (HR 1.05, 95%CI 1.017 - 1.085, P=0.0029) and pre-transplant ALT level (HR 1.004, 95%CI 1.001 - 1.007, P=0.0042) were associated with shorter time to recurrence. The recipient IL28B C allele remained an independent predictor of delayed recurrence of hepatitis C at 5 years: HR 0.632, 95% CI 0.466 - 0.856, P=0.0031 (Table 3). Pre-transplant MELD score and pre-transplant ALT level were both associated with shorter time to recurrence at 5 years also. The relationship between recipient IL28B genotype and time to recurrence of hepatitis C was independent of donor IL28B genotype (recipient IL28B genotype, P = 0.030 and P=0.015 when donor IL28B genotype was forced into the 2yr and 5yr models).
Table 3.
Survival analysis for recurrent hepatitis C
| Multivariate Cox regression analysis assessing factors predictive of recurrent chronic hepatitis C at 2 years | ||||
|---|---|---|---|---|
| HCV recurrence – 2yrs | Hazard Ratio | 95% CI | P-value | |
| Recipient IL28B genotype | ||||
| Overall (additive genetic model) | 0.619 | 0.434 | 0.883 | 0.0081 |
| CC vs TT | 0.383 | 0.190 | 0.775 | 0.0076 |
| CT vs TT | 0.599 | 0.334 | 1.073 | 0.0850 |
| Pre-transplant MELD score | 1.050 | 1.017 | 1.085 | .0029 |
| Pre-transplant ALT level (IU/mL) | 1.004 | 1.001 | 1.007 | 0.0042 |
| Multivariate Cox regression analysis assessing factors predictive of recurrent chronic hepatitis C virus at 5 years | ||||
|---|---|---|---|---|
| HCV recurrence – 5yrs | Hazard Ratio | 95% CI | P-value | |
| Recipient IL28B genotype | ||||
| Overall (additive genetic model) | 0.632 | 0.466 | 0.856 | 0.0031 |
| CC vs TT | 0.392 | 0.216 | 0.711 | 0.0021 |
| CT vs TT | 0.561 | 0.333 | 0.946 | 0.0301 |
| Pre-transplant MELD score | 1.035 | 1.004 | 1.067 | 0.0260 |
| Pre-transplant ALT level (IU/mL) | 1.004 | 1.001 | 1.007 | 0.0041 |
Among patients for whom donor liver IL28B genotype was available, recurrent hepatitis C was diagnosed in 85/172 (49%) at 2 years post-OLT, and in 114/172 (66%) at 5 years. Donor IL28B genotype was not associated with time to recurrence of hepatitis C (Log Rank P= 0.5566 and 0.3369, for 2 and 5 year survival analyses, respectively).
IL28B genotype and treatment response for recurrent hepatitis C
Analysis of the relationship between IL28B genotype and SVR was limited to patients for whom both recipient and donor IL28B genotype was available. 65 patients received antiviral therapy for recurrent hepatitis C. 50 patients were treated with pegIFN and 15 with standard IFN (77%). 57 (92%) received combination therapy with ribavirin. Ribavirin starting dose was titrated to renal function. Five patients could not be evaluated for SVR: 1 patient was recently treated and had not reached the end-of-follow-up, 3 died due to sepsis within six months of stopping treatment and before they reached the end-of-follow-up, and 1 patient completed their therapy in another center. The relationship between IL28B genotype and treatment outcome was therefore investigated in 60 patients.
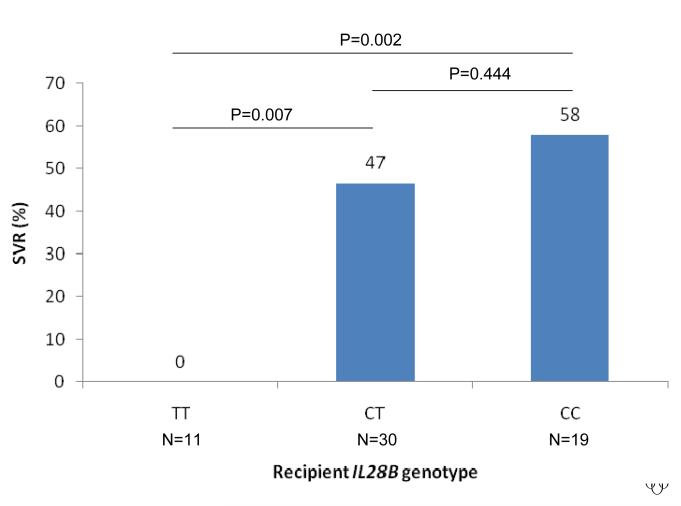
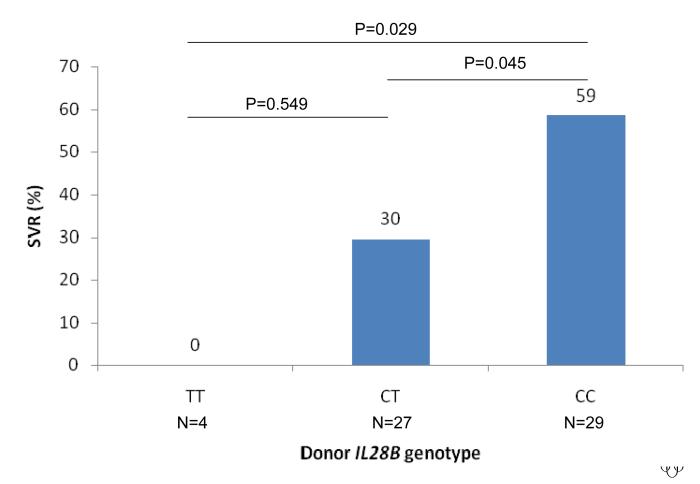
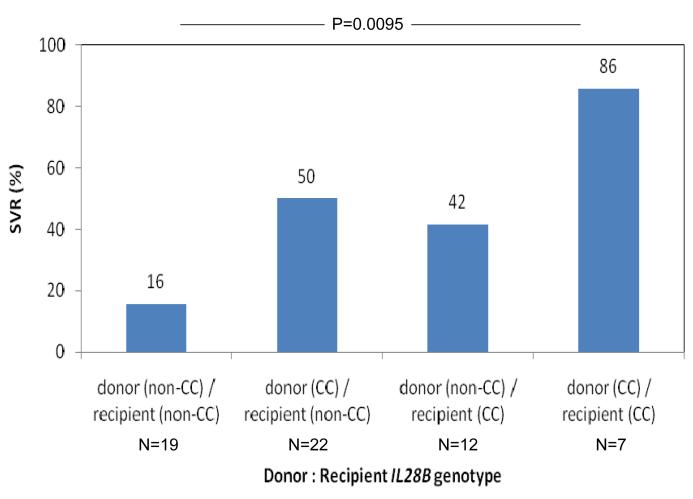
The rate of SVR was strongly associated with the IL28B genotype of both the recipient and donor liver. The rate of SVR according to recipient IL28B genotype was 58% vs 47% vs 0% for CC vs CT vs TT, OR = 3.43, 95%CI 1.42 - 8.3, P=0.0062 (Figure 1A). The rate of SVR according to donor genotype was 59% vs 30% vs 0% for CC vs CT vs TT, OR = 4.00, 95%CI = 1.46 - 10.98, P=0.0071 (Figure 1B). Notably, no patient with either recipient or donor TT genotype attained SVR (n=14). SVR was independently associated with both recipient and donor IL28B genotype in a regression model that included both as predictors (P=0.0026 and P=0.0030 respectively). We then considered treatment outcome according to combined recipient : donor IL28B genotype pairing (CC vs non-CC genotype, given the primary results and data from the non-transplant literature that shows that the major benefit of the IL28B polymorphism occurs in CC homozygotes 9, 10). SVR was lowest in non-CC recipients of a non-CC donor liver, increased if either the recipient or the donor was CC at the IL28B polymorphism and was maximal in the setting of CC recipients of a CC liver (SVR rates 3/19 (16%) vs 11/22 (50%) / 5/12 (42 %) vs 6/7 (86%), P=0.0095, Figure 1C). No other clinical / biochemical variable was associated with virological clearance in this cohort.
Figure 1.
Sustained virological response rates according to IL28B genotype are shown. The rate of SVR was strongly associated with the IL28B genotype of both the recipient and donor liver. The rate of SVR according to recipient IL28B genotype was 58% vs 47% vs 0% for CC vs CT vs TT, OR = 3.43, 95%CI 1.42 - 8.3, P=0.0062 (Figure 1A). The rate of SVR according to donor genotype was 59% vs 30% vs 0% for CC vs CT vs TT, OR = 4.00, 95%CI = 1.46 - 10.98, P=0.0071 (Figure 1B). SVR was lowest in non-CC recipients of a non-CC donor liver, increased if either the recipient or the donor was CC at the IL28B polymorphism and was maximal in the setting of CC recipients of a CC liver (SVR rates 3/19 (16%) vs 11/22 (50%) / 5/12 (42 %) vs 6/7 (86%), P=0.0095, Figure 1C).
Histology
Recipient IL28B CC genotype was associated with significantly less fibrosis formation in the early post-transplant period than non-CC genotypes. 172 patients underwent a biopsy at year 1. Twenty-six had genotype TT, 48 had genotype CC, 80 had the heterogeneous CT genotype and in 18 the IL28B genotype could not be determined.
Fibrosis
At one year posttransplantation, when biopsy data were most complete, 9/28 (32%) patients with recipient IL28B TT genotype had fibrosis stage 2 or higher, compared to 6/52 (12%) patients with genotype CC and 20/80 (25%) patients with the heterogeneous CT genotype (Pearson Chi-square p=0.024 for the comparison CC versus TT). Donor IL28B genotype was not significantly associated with fibrosis at one year.
Inflammation
Allograft inflammation at 1yr, measured by histologic activity index (HAI), did not vary significantly with donor or recipient genotype (Mann-Whitney TT versus CC p= 0.430). HAI was higher, but not significantly so, at the time of recurrence of HCV in recipients with a recipient IL28B genotype CC: 5.0 (range 3.0-7.0), CT 4.0 (range 3.0-6.0) and TT 3.0 (2.5-4.0), Kruskal-Wallis (overall) p=0.165.
Transaminases
ALT levels were lower for recipient IL28B genotype CC than for TT at 3-6 months post-OLT (138 U/l for TT, 93 U/l for CT, and 60 U/l for CC; Mann Whitney p=0.030 for CC versus TT). Although numerically higher among patients with donor CC genotype, median ALT levels did vary significantly with donor IL28B genotype (median ALT at 3-6 months post-OLT: 126 U/l for CC 94 U/l for TT, and 74 U/l for CT at 3 months post-OLT, Mann Whitney p=0.580 for CC versus TT).
Mortality and Graft failure
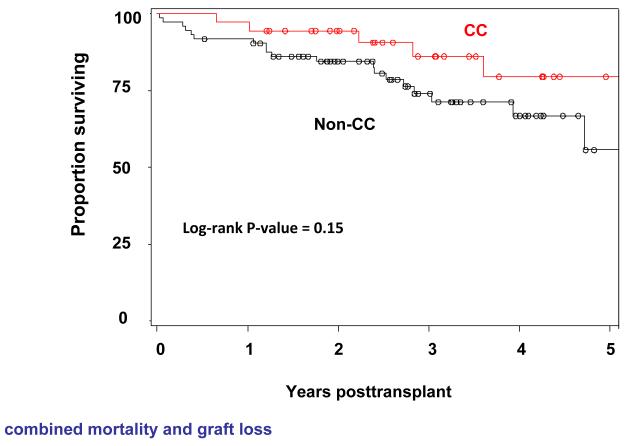
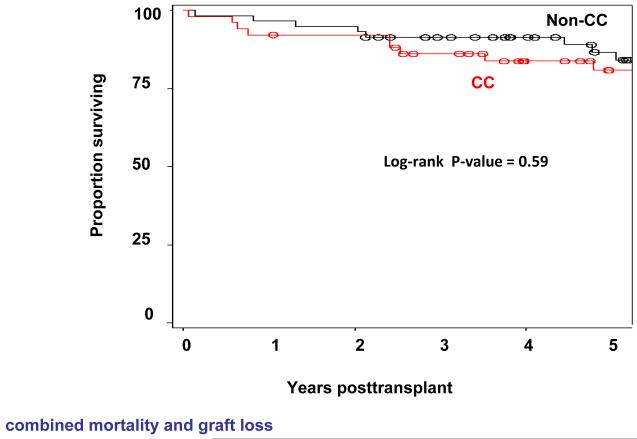
There was no statistically significant difference in overall graft survival according to recipient IL28B polymorphism (overall 5-year graft survival (n=118): 91% vs 76% vs 84% for CC vs CT vs TT genotypes (P=0.2168)). There was also no significant effect of donor IL28B genotype on overall graft survival (5-Year graft survival (n=124): 79% vs 84% vs 81% for CC vs CT vs TT genotype (P=0.6977)). Neither recipient nor donor liver IL28B genotype was found to be significantly associated with liver-related mortality at 5 years (P=0.3956 and P=0.2418, respectively)(Figure 2A and 2B).
Figure 2.
Overall survival (combined mortality and graft loss) according to IL28B recipient (Figure 2A) and donor (Figure 2B) genotype is shown. There was no significant effect of recipient or donor IL28B genotype on overall survival (P=0.15 for recipient and P=0.59 donor IL28B genotype).
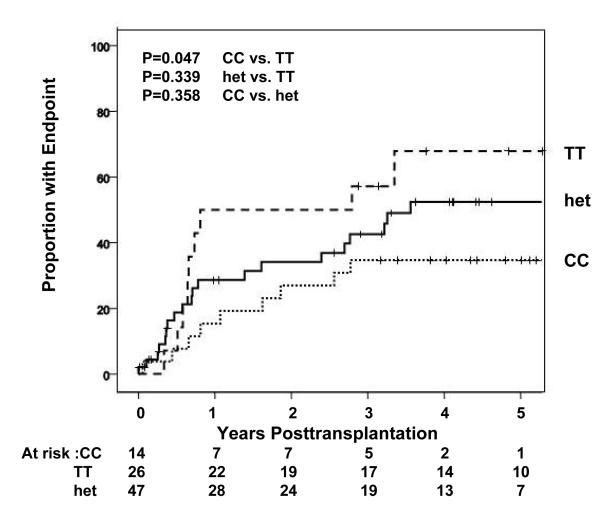
An analysis was also performed of the association of IL28B genotype with the frequency of a composite endpoint consisting of: histological evidence of cirrhosis, liver related death/retransplantation and fibrosis stage >/=2. The analysis was censored for antiviral therapy. This clinical composite endpoint was significantly associated with recipient and donor, IL28B genotype (p=0.047 and 0.040 for recipient and donor CC vs. TT genotypes respectively)(Figure 3).
Figure 3.
Occurrence over time of the combined endpoint (fibrosis stage (FS) >/=2, retransplantation and/or liver related death is shown (percent experiencing endpoint shown on y-axis) according to recipient IL28B genotype are shown. The analysis was censored for antiviral therapy. The clinical composite endpoint was significantly associated with recipient and donor, IL28B genotype (p=0.047 and 0.040 for recipient and donor CC vs. TT genotypes respectively).
Discussion
This study reports the association between IL28B genotype and virological treatment response and clinical outcome in HCV patients following liver transplantation. This unique cohort allowed interrogation of the respective roles of the IL28B genotype of hepatocytes (donor) and non-parenchymal cells of extra-hepatic origin (recipient). We identified important roles for both donor and recipient IL28B genotype in determining treatment outcome. The data also suggest that recipient IL28B genotype may determine the severity of histological recurrence of hepatitis C as indicated by progressive fibrosis. These findings have potentially important implications for the management of HCV following liver transplantation.
The frequency of the CC variant in the transplant recipients was significantly lower than that in the non-HCV donor livers. This is consistent with a role for the CC variant in spontaneous clearance of HCV, with enrichment for the non-CC variants in the chronic hepatitis C population. Indeed, a role for the CC variant in promoting natural clearance has recently been established 6, 7. Patients with the non-CC genotypes are also more likely to be prior non-responders to IFN-based therapies before proceeding to liver failure and transplantation.
IL28B polymorphism, previously associated with treatment response in the non-transplant setting 4, 5, 7, 9, 10, strongly predicted for increased rate of SVR in the current cohort. Recipient and donor IL28B genotype were both independently associated with higher rates of SVR. Compared to the patients with matched recipient: donor non-CC variants, SVR rate was higher in patients with either a donor or recipient CC variant, and highest in patients with matched donor and recipient CC variants. No patient in which either the recipient or donor IL28B genotype was TT attained an SVR. The low SVR rates associated with antiviral therapy in patients with IL28B genotype TT suggests that interferon and ribavirin based treatment might be avoided until more effective treatment protocols are developed. IL28B genotyping of donors and recipients is likely to be cost effective in liver transplant patients. In the same vein, because SVR rates of up to 80% can be achieved, recipients with. IL28B CC donor or recipient genotype should be considered for antiviral treatment if they have not already been treated.
The associations between IL28B genotype, antiviral treatment outcome, time to histological recurrence and more advanced fibrosis did not translate to a statistically significant benefit for either liver-related or overall survival. Trends for an association of recipient IL28B genotype were seen, however, for both overall and liver related survival (P=0.11 and P=0.18, respectively for CC vs non-CC genotypes). Our sample size may have been too small to detect an effect of IL28B genotype on these important individual outcomes. However, the frequency of a composite endpoint consisting of histological evidence of cirrhosis, liver related death/retransplantation and fibrosis stage >/=2 was significantly associated with recipient and donor IL28B genotype (p=0.047 and 0.040 for recipient and donor CC vs. TT genotypes respectively). As previously observed, antiviral therapy for HCV was strongly associated with survival benefit in this cohort. It remains possible that IL28B genotype may be relevant to survival, through its association with IFN responsiveness and sustained virological clearance, but that we were underpowered to detect such an effect in this cohort, of which only a minority received treatment. The very high SVR rates (>80%) seen in patients with IL28B CC donor and recipient genotype raises the possibility that the impact of recurrence of HCV might be reduced by preferentially allocating IL28B CC donors to IL28B non-CC genotype recipients. There are many practical and theoretical considerations that would need to be addressed before such a change to organ allocation policy could be considered, but the results of this study raise the theoretical possibility of improving the utility of liver transplantation among recipients with HCV infection.
The data raises the question of whether pre-transplant determination of IL28B genotype has consequences for the further management of HCV patients with end-stage liver disease. Although patients with TT genotype experienced earlier histological recurrence of hepatitis C, more advanced fibrosis, reduced SVR-rates to subsequent pegIFN treatment, recipient IL28B genotype was not an important predictor of graft survival in our cohort. Therefore, in our opinion, liver transplantation should not be withheld from patients with this genotype. The data also suggest that IL28B genotype may be clinically useful in selecting patients who have the best risk:benefit ratio for IFN-based treatment post-OLT. This association between recipient / donor IL28B genotype, treatment response and graft outcome will need to be confirmed in larger prospective studies.
The treatment response data also allow speculation about the biology of the association between IL28B polymorphism and pegIFN/RBV responsiveness. That both donor and recipient genotype were important suggests that the IL28B polymorphism may be associated with both hepatocyte (innate) and non-parenchymal cell (innate and / or adaptive) immune mechanisms of IFN effect. This would be consistent with recent data suggesting that phase 1 decline on-treatment, reflecting virion production / release, is dramatically increased in patients with the good response variant, but that an effect on phase 2 decline, reflecting infected cell loss due to adaptive immune mechanisms, is also present 11. The molecular biology underlying these observations will be an important area for future translational research.
The association between the recipient CC IL28B genotype and delayed time to diagnosis of HCV recurrence is interesting. HCV recurrence was defined in this cohort as the combination of virological recurrence and histological disease. The results suggest that recipient, but not donor, IL28B genotype influences the progression from virological recurrence to histological disease. As the key histological marker of recurrence was a typical portal and/or lobular lymphocytic infiltrate (of extrahepatic (recipient) cell origin), we speculate that recipient IL28B genotype may play a role in regulating the HCV-specific, HLA-independent,12, 13 adaptive immune response, either at the pattern recognition/signal transduction (dendritic cell) step or the effector (T lymphocyte, plasma cell) step. IL28 is known to induce toll-like receptor 7 and 8 expression and expression of HLA,14, 15 potential mechanisms for variation in immune response with IL28B genotype.
The protein product of IL28B is IFN-λ-3, one of the three members of the recently described type 3 IFN family16, 17. Type 3 IFN are secreted in response to stimuli that also trigger type 1 IFN, and activate the common JAK/STAT signaling pathway. A key distinction lies in the restricted expression profile of the unique IFN-λ-receptor, present on hepatocytes but low/absent on CD34+ bone marrow progenitor cells 16. IFN-λ inhibits HCV in vitro,18 and antiviral activity of recombinant IFN-λ-1 (IL29) has recently been confirmed in HCV-1 patients.19 The molecular consequences of the IL28B polymorphism, and the mechanistic explanation for the relationship with IFN responsiveness as yet remain unclear.
There are a number of limitations to this study. Firstly, decisions about treatment assignment were not randomized, but rather made by individual clinicians on a case-by-case basis, and selection bias cannot be excluded in both the analyses of treatment outcome as well as survival. Secondly, treatment regimens were not uniform, although there were no obvious differences according to recipient : donor genotype pairs. Lastly, although the cohort is larger than most studies of HCV post-OLT, power to detect smaller effects on survival was low. The data should therefore be considered hypothesis generating.
In conclusion, the data suggest that recipient IL28B genotype is associated with more rapid histological recurrence of HCV. Recipient and donor liver IL28B genotype are strongly and independently associated with IFN-based treatment response in patients post-OLT. Treatment was safe, and has previously been associated with improved graft survival in this cohort. The data therefore support the preferential allocation of CC donor livers to patients with HCV infection. Prospective validation in larger multi-center cohorts is warranted.
Supplementary Material
Acknowledgments
This work has been supported by Public Health Service grant GCRC RR00585.
Footnotes
Post script: One of the authors, JM, has a significant financial interest in IL28B genotyping. To manage this potential conflict of interest, JM (and the entire Duke team) was blinded to all posttransplant outcomes at the time of genotyping. Only after all genotyping was performed and the results disclosed to Mayo investigators were outcomes metrics disclosed to the Duke collaborators, including JM. No one at Mayo has any financial interest in IL28B testing.
References
- 1.Sallie R, Cohen AT, Tibbs CJ, Portmann BC, Rayner A, O’Grady JG, Tan KC, Williams R. Recurrence of hepatitis C following orthotopic liver transplantation: a polymerase chain reaction and histological study. J Hepatol. 1994;21:536–42. doi: 10.1016/s0168-8278(94)80098-7. [DOI] [PubMed] [Google Scholar]
- 2.Angelico M, Petrolati A, Lionetti R, Lenci I, Burra P, Donato MF, Merli M, Strazzabosco M, Tisone G. A randomized study on Peg-interferon alfa-2a with or without ribavirin in liver transplant recipients with recurrent hepatitis C. J Hepatol. 2007;46:1009–17. doi: 10.1016/j.jhep.2006.12.017. [DOI] [PubMed] [Google Scholar]
- 3.Ge D, Fellay J, Thompson AJ, Simon JS, Shianna KV, Urban TJ, Heinzen EL, Qiu P, Bertelsen AH, Muir AJ, Sulkowski M, McHutchison JG, Goldstein DB. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature. 2009;461:399–401. doi: 10.1038/nature08309. [DOI] [PubMed] [Google Scholar]
- 4.Tanaka Y, Nishida N, Sugiyama M, Kurosaki M, Matsuura K, Sakamoto N, Nakagawa M, Korenaga M, Hino K, Hige S, Ito Y, Mita E, Tanaka E, Mochida S, Murawaki Y, Honda M, Sakai A, Hiasa Y, Nishiguchi S, Koike A, Sakaida I, Imamura M, Ito K, Yano K, Masaki N, Sugauchi F, Izumi N, Tokunaga K, Mizokami M. Genome-wide association of IL28B with response to pegylated interferon-alpha and ribavirin therapy for chronic hepatitis C. Nat Genet. 2009 doi: 10.1038/ng.449. [DOI] [PubMed] [Google Scholar]
- 5.Suppiah V, Moldovan M, Ahlenstiel G, Berg T, Weltman M, Abate ML, Bassendine M, Spengler U, Dore GJ, Powell E, Riordan S, Sheridan D, Smedile A, Fragomeli V, Muller T, Bahlo M, Stewart GJ, Booth DR, George J. IL28B is associated with response to chronic hepatitis C interferon-alpha and ribavirin therapy. Nat Genet. 2009 doi: 10.1038/ng.447. [DOI] [PubMed] [Google Scholar]
- 6.Thomas DL, Thio CL, Martin MP, Qi Y, Ge D, O’Huigin C, Kidd J, Kidd K, Khakoo SI, Alexander G, Goedert JJ, Kirk GD, Donfield SM, Rosen HR, Tobler LH, Busch MP, McHutchison JG, Goldstein DB, Carrington M. Genetic variation in IL28B and spontaneous clearance of hepatitis C virus. Nature. 2009;461:798–801. doi: 10.1038/nature08463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rauch A, Kutalik Z, Descombes P, Cai T, Di Iulio J, Mueller T, Bochud M, Battegay M, Bernasconi E, Borovicka J, Colombo S, Cerny A, Dufour JF, Furrer H, Gunthard HF, Heim M, Hirschel B, Malinverni R, Moradpour D, Mullhaupt B, Witteck A, Beckmann JS, Berg T, Bergmann S, Negro F, Telenti A, Bochud PY. Genetic variation in IL28B is associated with chronic hepatitis C and treatment failure: a genome-wide association study. Gastroenterology. 2010;138:1338–45. 1345, e1–7. doi: 10.1053/j.gastro.2009.12.056. [DOI] [PubMed] [Google Scholar]
- 8.Veldt BJ, Poterucha JJ, Watt KD, Wiesner RH, Hay JE, Kremers WK, Rosen CB, Heimbach JK, Charlton MR. Impact of pegylated interferon and ribavirin treatment on graft survival in liver transplant patients with recurrent hepatitis C infection. Am J Transplant. 2008;8:2426–33. doi: 10.1111/j.1600-6143.2008.02362.x. [DOI] [PubMed] [Google Scholar]
- 9.Thompson AJ, Muir A, Sulkowski M, et al. IL28B Polymorphism Improves Viral Kinetics and Is the Strongest Pre-treatment Predictor of SVR in HCV-1 Patients. Gastroenterology. 2010 Apr 15; doi: 10.1053/j.gastro.2010.04.013. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 10.McCarthy JJ, Li JH, Thompson A, Suchindran S, Lao XQ, Patel K, Tillmann HL, Muir AJ, McHutchison JG. Replicated Association Between an Interleukin-28B Gene Variant and a Sustained Response to Pegylated Interferon and Ribavirin. Gastroenterology. 2010 Feb 19; doi: 10.1053/j.gastro.2010.02.009. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neumann A, Bibert S, Haagmans B, Soulier A, Negro F, Lagging M, Ferrari C, Zeuzem S, Pawlotsky J-M, Schalm S, Bochud P-Y, group D-H IL28B POLYMORPHISM IS SIGNIFICANTLY CORRELATED WITH IFN ANTI-VIRAL EFFECTIVENESS ALREADY ON FIRST DAY OF PEGYLATED INTERFERON-A AND RIBAVIRIN THERAPY OF CHRONIC HCV INFECTION. Journal of Hepatology. 2010;52(Suupl 1):S468. (2011) [Google Scholar]
- 12.Prigozy TI, Naidenko O, Qasba P, Elewaut D, Brossay L, Khurana A, Natori T, Koezuka Y, Kulkarni A, Kronenberg M. Glycolipid antigen processing for presentation by CD1d molecules. Science. 2001;291:664–7. doi: 10.1126/science.291.5504.664. [DOI] [PubMed] [Google Scholar]
- 13.Balato A, Unutmaz D, Gaspari AA. Natural killer T cells: an unconventional T-cell subset with diverse effector and regulatory functions. J Invest Dermatol. 2009;129:1628–42. doi: 10.1038/jid.2009.30. [DOI] [PubMed] [Google Scholar]
- 14.Zhu H, Butera M, Nelson DR, Liu C. Novel type I interferon IL-28A suppresses hepatitis C viral RNA replication. Virol J. 2005;2:80. doi: 10.1186/1743-422X-2-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Siren J, Pirhonen J, Julkunen I, Matikainen S. IFN-alpha regulates TLR-dependent gene expression of IFN-alpha, IFN-beta, IL-28, and IL-29. J Immunol. 2005;174:1932–7. doi: 10.4049/jimmunol.174.4.1932. [DOI] [PubMed] [Google Scholar]
- 16.Kotenko SV, Gallagher G, Baurin VV, Lewis-Antes A, Shen M, Shah NK, Langer JA, Sheikh F, Dickensheets H, Donnelly RP. IFN-lambdas mediate antiviral protection through a distinct class II cytokine receptor complex. Nat Immunol. 2003;4:69–77. doi: 10.1038/ni875. [DOI] [PubMed] [Google Scholar]
- 17.Sheppard P, Kindsvogel W, Xu W, Henderson K, Schlutsmeyer S, Whitmore TE, Kuestner R, Garrigues U, Birks C, Roraback J, Ostrander C, Dong D, Shin J, Presnell S, Fox B, Haldeman B, Cooper E, Taft D, Gilbert T, Grant FJ, Tackett M, Krivan W, McKnight G, Clegg C, Foster D, Klucher KM. IL-28, IL-29 and their class II cytokine receptor IL-28R. Nat Immunol. 2003;4:63–8. doi: 10.1038/ni873. [DOI] [PubMed] [Google Scholar]
- 18.Robek MD, Boyd BS, Chisari FV. Lambda interferon inhibits hepatitis B and C virus replication. J Virol. 2005;79:3851–4. doi: 10.1128/JVI.79.6.3851-3854.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shiffman ML, et al. PEG-IFN-λ: Antiviral Activity and Safety Profile in a 4-Week Phase 1b Study in Relapsed Genotype 1 Hepatitis C Infection. J Hepatol. 2009;50(Suppl. 1):s237. (A643) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.