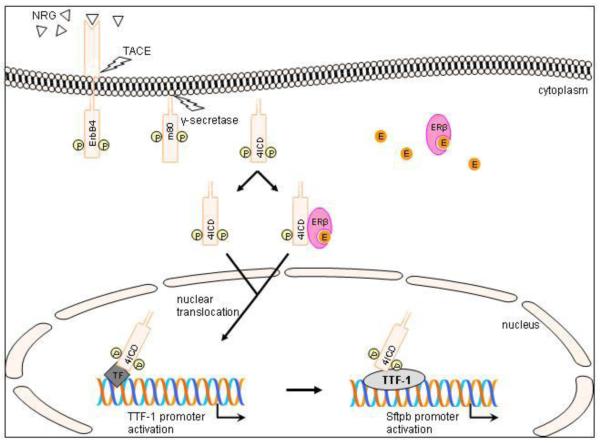
Figure 9. Schematic model of ErbB4 signaling in type II epithelial cells proposed by the authors.
Neuregulin (NRG)-binding leads to activation and proteolytic cleavage of the phosphorylated (P) ErbB4 receptor [24, 25]. The remaining membrane bound part (m80) is further processed by γ-secretase. The resulting 80kDa ErbB4 intracellular domain (4ICD) translocates to the nucleus [26] and enters via its nuclear localization sequence (NLS) [13, 24] or NLS independently using an estrogen (E) activated estrogen receptor β (ERβ) as a nuclear shuttle [28]. In our proposed model, 4ICD leads to TTF-1 promoter activation by binding via an additional transcription factor (TF) or linker to the promoter. This newly formed complex than binds and activates the Sftpb promoter to stimulate Sftpb expression.