Abstract
Background
The chemotherapy resistance of non-small cell lung cancer (NSCLC) remains a clinic challenge and is closely associated with several biomarkers including epidermal growth factor receptor (EGFR) ( Drugs 72(Suppl 1):28–36, 012.), p53 ( Med Sci Monit 11(6):HY11–HY20, 2005.) and excision repair cross complementing gene 1 (ERCC1) ( J Thorac Oncol 8(5):582–586, 2013.). Fluorodeoxyglucose positron emission tomography (FDG–PET) is the best non-invasive surrogate for tumor biology with the maximal standardized uptake values (SUVmax) being the most important paradigm. However, there are limited data correlating FDG-PET with the chemotherapy resistant tumor markers. The purpose of this study was to determine the correlation of chemotherapy related tumor marker expression with FDG–PET SUVmax in NSCLC.
Methods
FDG–PET SUVmax was calculated in chemotherapy naïve patients with NSCLC (n = 62) and immunohistochemical analysis was performed for EGFR, p53 or ERCC1 on the intraoperative NSCLC tissues. Each tumor marker was assessed independently by two pathologists using common grading criteria. The SUVmax difference based on the histologic characteristics, gender, differentiation, grading and age as well as correlation analysis among these parameters were performed. Multiple stepwise regression analysis was further performed to determine the primary predictor for SUVmax and the receiver operating characteristics (ROC) curve analysis was performed to detect the optimized sensitivity and specificity for SUVmax in suggesting chemotherapy resistant tumor markers.
Results
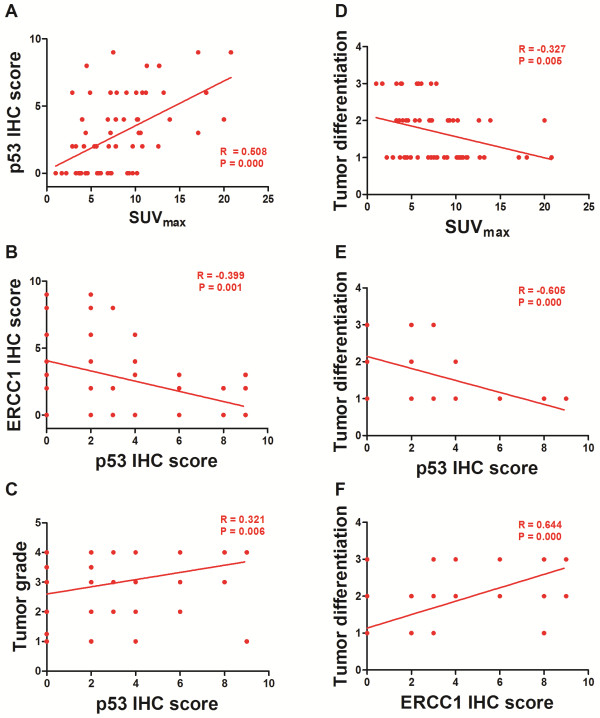
The significant tumor type (P = 0.045), differentiation (P = 0.021), p53 (P = 0.000) or ERCC1 (P = 0.033) positivity dependent differences of SUVmax values were observed. The tumor differentiation is significantly correlated with SUVmax (R = -0.327), tumor size (R = -0.286), grading (R = -0.499), gender (R = 0.286) as well as the expression levels for p53 (R = -0.605) and ERCC1 (R = -0.644). The expression level of p53 is significantly correlated with SUVmax (R = 0.508) and grading (R = 0.321). Furthermore, multiple stepwise regression analysis revealed that p53 expression was the primary predictor for SUVmax. When the cut-off value of SUVmax was set at 5.15 in the ROC curve analysis, the sensitivity and specificity of SUVmax in suggesting p53 positive NSCLC were 79.5% and 47.8%, respectively.
Conclusion
The current study suggests that SUVmax of primary tumor on FDG-PET might be a simple and good non-invasive method for predicting p53-related chemotherapy resistance in NSCLC when we set the cu-off value of SUVmax at 5.15.
Keywords: Non–small cell lung cancer, Tumor markers, Fluorodeoxyglucose positron emission tomography (FDG–PET)
Background
Lung cancer is the most frequently diagnosed cancer and leads to the most cancer mortality worldwide which accounts for almost 1.3 million deaths a year [1]. Nearly 85% of lung cancer cases are represented by non-small cell lung cancer (NSCLC) with the early diagnosis and effective therapy being two main issues [2].
Although significant therapeutic advances have been achieved, poor prognosis and short survival time of patients, as well as the limited value of any sort of conventional therapy are the current dilemma for NSCLC therapy [3]. Platinum-based adjuvant chemotherapy is usually recommended after surgical resection of NSCLC with good performance status and completely resected stage IB-IIIA disease [4]. Such combinational therapy did improve the survival for some patients with early-stage NSCLC [5-7]. However, a large population remains resistant to chemotherapy [8], which has also been confirmed in NSCLC tumor culture study [9]. Increasing evidences advocate the concept that some molecular markers including epidermal growth factor receptor (EGFR) [10], p53 [11] and excision repair cross complementing gene 1 (ERCC1) [12] are associated with chemotherapy resistance in NSCLC. Clarifying the relationship of these molecular markers with noninvasive diagnostic methods is important for the planning of therapeutic strategy.
Fluorodeoxyglucose positron emission tomography (FDG–PET) has become an important non-invasive tool for diagnosing and staging in NSCLC. FDG–PET maximal standardized uptake values (SUVmax) of primary tumors have been shown to correlate with both stage and nodal disease in NSCLC [13]. Several studies have reported the relationship between the SUVmax and the expression levels of some biomarkers, such as Glut 1[14], COX-2[15], Ki-67[16] and vascular endothelial growth factor (VEGF) [17]. Thus we hypothesized that the SUVmax of FDG has some close relationship with the chemotherapy resistance associated biomarkers and can serve as a tool to predict some specific chemotherapy résistance for better planning the individualized therapeutic strategy.
The purpose of this study is to examine the relationship between the expressions of chemotherapy resistance related tumor markers and FDG–PET. The SUVmax difference based on the histologic characteristics, gender, differentiation, grading and age as well as correlation analysis among these parameters were performed. Multiple stepwise regression analysis was further performed to determine the primary predictor for SUVmax. Collectively, the current study will offer insight into the relationships between expression of these specific tumor markers and FDG–PET in NSCLC.
Methods
Study population
Sixty-two patients with diagnosed NSCLC by biopsy (38/62) or operation (24/62) who were naïve to chemotherapy from the cancer center of our hospital from January 1, 2011 to December 31, 2012 were enrolled in this study. The FDG-PET/CT was performed within one week before biopsy or operation. The histological type was determined according to the World Health Organization (WHO) criteria [18] and the tumor–node–metastasis (TNM) staging system was used according to the criteria in 2011.
Paraffin-embedded primary lung tumor samples were obtained from the pathological department of our hospital. All tissue sections were reviewed for histological type and graded by two pathologists blinded to FDG-PET results. Written informed consent was obtained from each enrolled patient for the study of the excised tissue. This study was conducted with the approval of the institutional ethics committee of the First Affiliated Hospital of Xi’an Jiaotong University.
18 F-FDG PET/CT
Patients were fasted for 6 hours prior to imaging. FDG-PET images were obtained at 40 min after FDG injection (3.7 MBq /kg) with a PET/CT system (GEMINI 64TF, Philips, Cleveland, USA). Non-contrast CT scan was performed prior to the PET scan with the multidetector spiral CT scanner. PET scan was performed immediately with an acquisition time of 2.0 min/bed position during shallow breathing with the scan field limited from head up to the upper tights. Diagnostic CT scan of chest with respiratory control was performed on the same PET/CT system. Co-registered images were displayed by means of SYNTEGRA software (Philips Medical Systems).
PET/CT images were evaluated by two nuclear physicians in a blinded manner. The SUVmax was determined by drawing region of interest (ROI) around the primary tumor on the transaxial slices, and calculated using the following equation: .
Immunohistochemical analysis
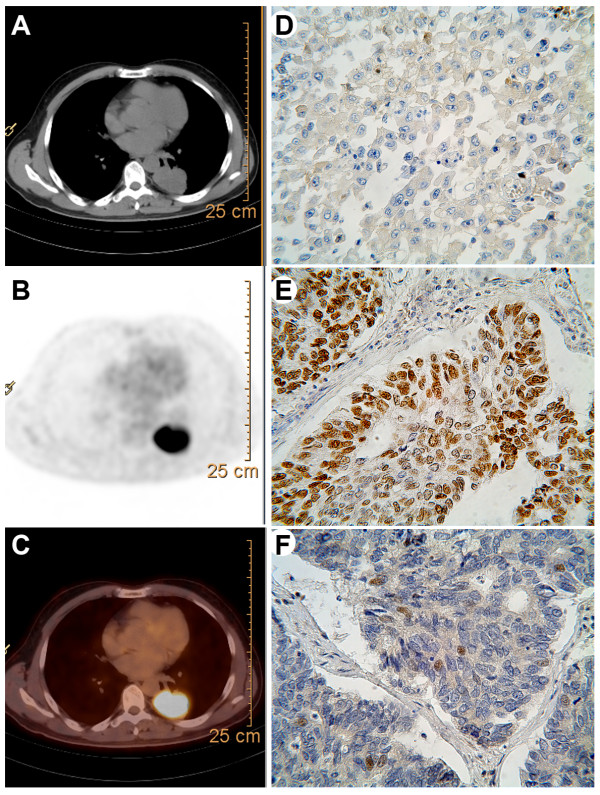
Immunohistochemical analysis was performed on paraffin-embedded lung cancer tissues. Information of the antibody, dilution and staining pattern were summarized in Table 1. The sections were examined by 2 investigators who had no knowledge of the corresponding clinical pathologic data. For p53 (Figure 1E) and ERCC1 (Figure 1F), nucleus and/or cytoplasm staining was considered positive. EGFR was considered positive when cell membrane and/or cytoplasm staining was observed (Figure 1D). Intensity of staining was scored as the following: 0 (no staining), 1+ (weak staining), 2 + (intermediate staining), 3 + (strong staining). The percentage of positive cells was scored as 0 (0%), 1 (1% to 9%), 2 (10% to 49%), and 3 (50% to 100%) for ERCC1 and p53. For EGFR, it is 0 (0%), 1 (1% to 9%), 2 (10% to 25%), and 3 (>25%). The immunohistochemistry (IHC) score (0 to 9) was defined according to the product intensity and percentage of positive cells. We categorized the patients into four groups according to IHC score (0, 1 to 3, 4 to 6, 7 to 9). The biomarkers expression was judged as positive when the IHC score was greater than or equal to 1 (groups 2, 3 and 4) (Figure 1D, E and F). EGFR, p53 and ERCC1 were positive in 43.5%, 62.9% and 67.7% NSCLCs.
Table 1.
Antibodies used for immunohistochemical analysis
| Antibody | Company | Catolog# | Clone | Dilution | Positive staining pattern |
|---|---|---|---|---|---|
| EGFR |
Invitrogen |
ZM-0083 |
31G7 |
1:100 |
Cytomembrane/Cytoplasm |
| p53 |
Invitrogen |
ZM-0408 |
BP53.12 |
1:50 |
Nucleus |
| ERCC1 | Invitrogen | ZM-0138 | 4 F9 | 1:50 | Nucleus/Cytoplasm |
EGFR = epidermal growth factor receptor; ERCC1 = excision repair cross complementing gene 1.
Figure 1.
Representative images of PET-CT and immunohistochemistry. Transaxial images of (A) diagnostic CT, (B) FDG-PET and (C) fusion of PET and CT images. Immunohistochemical stainings for (D) epidermal growth factor receptor (EGFR), (E) p53 and (F) excision repair cross complementing gene 1(ERCC1). (magnification, ×400).
Statistical analysis
Statistical analysis was performed using SPSS software, version 17.0 (SPSS Inc, Chicago, IL). The results were expressed as mean ± standard error mean (SEM). The age, tumor size, p53 positivity and ERCC1 positivity dependent differences were tested using student t-test or one way analysis of the variable (ANOVA) followed by LSD post hoc test. Spearman correlation analysis was used to determine the relationship between different parameters. To identify the primary predictor for SUVmax, multiple stepwise regression analysis was performed. Receiver operating characteristics (ROC) curve analysis was generated that maximized the sensitivity and the specificity and thus the accuracy for assessing a cut off value for SUVmax ratio. Differences were considered significant when the P value was less than 0.05.
Results
Clinical characteristics
The characteristics of the patients are summarized in Table 2. The patients’ age ranged from 33 to 81 years (median age, 62 years). There were 47 men (median age 65 years) and 15 women (median age 60 years) and there was no difference in ages of these 2 groups (P = 0.095). The median values of the SUVmax were 7.2 (range, 1 to 20.8), 7.8 (range, 2.2 to 20.8) and 5.7 (range, 1 to 17.1) in the total, male, and female populations, respectively. Histological type of NSCLC fell in adenocarcinoma (n = 40) and squamous cell carcinoma (n = 22). No significant difference in SUVmax of the groups with different age (P = 0.077), gender (P = 0.147) or tumor size (P = 0.064) was observed (Table 2, Figure 2).
Table 2.
Characteristics of the patients with SUV max
| Factor | All patients (n = 62) | SUV max (mean ± SEM) | P value |
|---|---|---|---|
| Age |
0.077 |
||
| < 60 |
25 |
6.81 ± 0.61 |
|
| ≥ 60 |
37 |
8.83 ± 0.83 |
|
| Gender |
0.147 |
||
| Male |
47 |
8.48 ± 0.64 |
|
| Female |
15 |
6.57 ± 1.13 |
|
| Tumor size (diameter) |
0.064 |
||
| < 3 cm |
14 |
6.10 ± 0.80 |
|
| ≥ 3 cm |
48 |
8.58 ± 0.67 |
|
| Tumor differentiation |
0.021 |
||
| Well |
11 |
4.85 ± 0.69 |
|
| Moderate |
20 |
8.10 ± 1.81 |
|
| Poor |
31 |
9.09 ± 0.85 |
|
| Tumor type |
0.045 |
||
| Adenocarcinoma |
22 |
9.53 ± 1.01 |
|
| Squamous cell carcinoma |
40 |
7.19 ± 0.64 |
|
| Stage |
0.612 |
||
| I |
10 |
6.35 ± 1.77 |
|
| II |
9 |
8.27 ± 2.76 |
|
| III |
19 |
8.06 ± 0.77 |
|
| IV | 24 | 8.59 ± 1.01 | |
Figure 2.
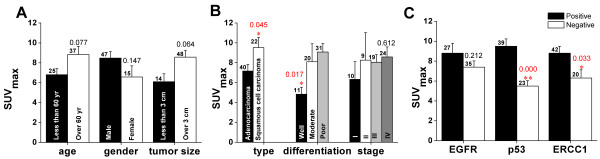
Group difference of SUVmax. The SUVmax differences among the patients with different (A) age, gender and tumor size; (B) cancer type, differentiation, stage as well as (C) expression of 3 biomarkers.** P < 0.01; * P < 0.05; # in (B)*P < 0.05 vs well-differentiation group; SUVmax = maximal standardized uptake value.
The age, tumor size, p53 positivity and ERCC1 positivity dependent differences in SUVmax
Student t-test and one way ANOVA were performed to determine the parameter based group differences in SUVmax (Table 2, Figure 2). In the current study, Student t-test revealed significantly higher SUVmax in the patient population with squamous cell carcinoma (P = 0.045), p53 positive (P = 0.000) or ERCC positive cancers (P = 0.033), respectively.
One way ANOVA revealed significant difference in the mean SUVmax of NSCLC with different differentiation [F (2,61) = 4.126, P = 0.021]. LSD post hoc test revealed that the difference was derived from the significantly higher SUVmax from the NSCLC patients with poor (P = 0.017) differentiation. There was no significant difference in the SUVmax from poorly and moderately differentiated tumors (P = 1) or moderately and well differentiated tumors (P = 0.132). On the other hand, no difference in the SUVmax of patients at different clinical stages [F (3,61) = 0.608, P = 0.612] was observed (Figure 2).
Correlationship analysis among the parameters
Table 3 demonstrated the correlationship analysis among the parameters. SUVmax was significantly correlated with p53 IHC score (R = 0.508, P = 0.000, also see Figure 3A) or tumor differentiation (R = -0.327, P = 0.005, also see Figure 3D). Besides SUVmax, p53 IHC score was significantly correlated with ERCC1 IHC score (R = -0.399, P = 0.001, also see Figure 3B), tumor differentiation (R = -0.605, P = 0.000, also see Figure 3E) or clinical stage (R = 0.321, P = 0.006, also see Figure 3C). Furthermore, tumor differentiation was significantly correlated with other factors including ERCC1 IHC score (R = 0.644, P = 0.000, also see Figure 3F), tumor long axis (R = -0.323, P = 0.006), clinical stages (R = -0.499, P = 0.000) or gender (R = 0.286, P = 0. 013).
Table 3.
Correlation analysis among different parameters
| SUV max | p53 | ERCC1 | Tumor size | Long | Differentiation | Grading | Age | Gender | ||
|---|---|---|---|---|---|---|---|---|---|---|
|
Pearson correlation |
SUV
max
|
1.000 |
.508** |
-.067 |
.174 |
.206 |
-.327** |
.143 |
-.118 |
-.168 |
| |
p53 |
.508** |
1.000 |
-.399** |
.158 |
.196 |
-.605** |
.321** |
-.106 |
-.191 |
| |
ERCC1 |
-.067 |
-.399** |
1.000 |
-.181 |
-.175 |
.644** |
-.241* |
.093 |
.240* |
| |
tumorsize |
.174 |
.158 |
-.181 |
1.000 |
.920** |
-.286* |
-.017 |
.170 |
-.112 |
| |
long |
.206 |
.196 |
-.175 |
.920** |
1.000 |
-.323** |
-.069 |
.127 |
-.077 |
| |
differentiation |
-.327** |
-.605** |
.644** |
-.286* |
-.323** |
1.000 |
-.499** |
.197 |
.286* |
| |
grading |
.143 |
.321** |
-.241* |
-.017 |
-.069 |
-.499** |
1.000 |
-.048 |
-.206 |
| |
age |
-.118 |
-.106 |
.093 |
.170 |
.127 |
.197 |
-.048 |
1.000 |
-.030 |
| gender | -.168 | -.191 | .240 | -.112 | -.077 | .286* | -.206 | -.030 | 1.000 |
*, P < 0.05; **, P < 0.01.
Figure 3.
Correlationship analysis among the parameters. SUVmax was significantly correlated with p53 IHC score (A, R = 0.508, P = 0.000) or tumor differentiation (D, R = -0.327, P = 0.005). The IHC score of p53 was significantly correlated with that of ERCC1 (B, R = -0.399, P = 0.001), tumor differentiation (E, R = -0.605, P = 0.000) or clinical stage (C, R = 0.321, P = 0.006). Furthermore, tumor differentiation was significantly correlated with ERCC1 IHC score (F, R = 0.644, P = 0.000).
Based on the findings that p53 IHC level was closely related with SUVmax and ERCC1 positive tumors demonstrated significantly higher SUVmax, it is reasonable to hypothesize that SUVmax might be usable in predicting the p53 or ERCC1 related chemotherapy resistance. Thus, we performed the multiple stepwise regression analysis to determine which molecule is the primary predictor for SUVmax.
IHC score of p53 is the primary predictor for SUVmax
Employing the multiple stepwise regression model, we input the SUVmax as the dependent variable, all the other parameters including age, gender, tumor size, differentiation, clinical stage, IHC score for p53 and ERCC1 as the independent variables. Multiple stepwise regression analysis revealed that the adjusted R2 for p53 IHC score is 0.246 and the P value is 0.000 (Table 4, Additional file 1: Figure S1). This statistical finding strongly suggests that p53 IHC score is the primary predictor for SUVmax. In another word, the SUVmax reflects the expression level of p53, thus may offer useful information for the p53 related chemotherapy resistance.
Table 4.
Multiple stepwise regression analysis of primary predictor for SUV max
|
Model |
R |
R
2
|
Adjusted R
2
|
SE of the Estimate |
Change statistics |
||||
|---|---|---|---|---|---|---|---|---|---|
| R 2 Change | F Change | df1 | df2 | Sig. F Change | |||||
| MSR | .508 | .258 | .246 | 3.84094 | .258 | 20.562 | 1 | 59 | .000 |
Predictor: p53; Dependent Variable: SUVmax; MSR: Multiple Stepwise Regression; SUVmax = maximal standardized uptake value.
The SUVmax greater than 2.5 is often used as a cut-off value for malignancy. However it has been shown that there is a significant number of false positivity (due to inflammatory diseases) and false negativity (due to low-grade malignancies) in the evaluation of primary tumor [19]. A recent study suggested that the cut-off value of SUVmax larger than 5 leads to an optimized diagnosing sensitivity and specificity of NSCLC [20]. We thus investigated the sensitivity and specificity at these two cut-off values.
ROC curve analysis revealed that the area under the curve is 0.769 with the 95% confidence interval (CI) ranging from 0.654 to 0.884 (p = 0.000). When the cut-off value of SUVmax was set at 2.55, the sensitivity and specificity of suggesting p53 positive NSCLC were 100% and 13%, respectively. However, when we set the cut-off value of SUVmax at 5.15, the sensitivity and specificity of suggesting p53 positive NSCLC were 79.5% and 47.8%, respectively (Figure 4).
Figure 4.
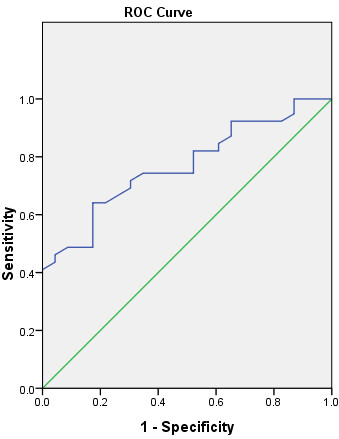
The receiver operating characteristics (ROC) curve for the optimal cut-off value of SUVmax in suggesting p53 positive NSCLC. Area under the curve: 0.769; 95% CI: 0.654 to 0.884; p = 0.000. A SUVmax ratio of 5.15 or lower suggests a NSCLC to be p53 positive with a sensitivity of 79.5% and specificity of 47.8%.
Discussion
FDG-PET, one of the current-available non-invasive imaging methods, has long been used to determine the enhanced metabolism in malignant tumor indicated by increased glucose uptake which is represented by an increased SUVmax. Our study offers further evidence that the SUVmax of FDG-PET may be a predicting parameter for some chemotherapy resistant NSCLCs, especially for the p53 or ERCC1 related chemotherapy resistance. Furthermore, SUVmax may be the most relevant parameter for p53 related chemotherapy which suggests the future clinical application to design the therapeutic plan.
EGFR is a cell surface receptor found primarily on cells with epithelial origin. EGFR overexpresses in both cell lines and samples of NSCLC, and contributes to the increased tumor proliferation, poor differentiation, higher incidence of metastases to lymph nodes and a worse prognosis [21]. Previous studies have demonstrated that expression status of EGFR can predict treatment response and survival benefit from the addition of cetuximab to first-line chemotherapy in patients with advanced NSCLC [11]. Taylor and colleagues [22] found that there was no correlation between SUVmax and EGFR expression in esophageal cancer specimens. Shimizu et al [15] reported that phosphorylated EGFR-positive cases showed higher SUVmax than negative cases in lung adenocarcinoma, but without statistical significance. Our finding is quite consistent with theirs in that there is no relationship between EGFR expression and SUVmax in NSCLCs. Furthermore, we did not reveal any difference in the SUVmax between adenocarcinoma and squamous cell carcinoma. Our study, together with previous one [15], suggests that FDG-PET may not be suitable for determining EGFR-related chemotherapy resistance or evaluating therapeutic effect of anti-EGFR treatment for NSCLCs.
The anti-cancer mechanism for the platinum compounds is to form adducts and covalent cross-links between DNA double strands and thus effectively block DNA replication and transcription. ERCC1 can recognize and remove these adducts and covalent cross-links, thus resistant to platinum agents [12]. A recent meta-analysis indicated that high ERCC1 level was a positive prognostic factor, being associated with shorter survival and lower response to platinum-based chemotherapy in advanced NSCLC patients [23]. Interestingly, we revealed that the SUVmax of ERCC1-positive cases were significantly higher than that of ERCC1-negative cases, there was statistical correlation between SUVmax and ERCC1 level, but failed to detect robust correlationship when the multiple stepwise regression was performed. It is still inconclusive whether SUVmax can be used to determine ERCC1 related chemotherapy resistance based on our current study.
As a tumor suppressor gene, p53 is capable of either arresting the cell cycle or inducing apoptosis. Tumors expressing p53 were less resistant to cisplatin, carboplatin, paclitaxel, and gemcitabine [10], probably due to the transcription of some MDR genes in these tumors [24]. A previous study suggested that there was no association between p53 expression and FDG uptake in 23 resected NSCLCs [25]. This is inconsistent with our current finding that the mean SUVmax of p53-positive cases was statistically higher than that of p53-negative cases. Besides, we also offered evidence that p53 expression is the primary predicting factor for the SUVmax. Our findings lead to the concept that FDG-PET can be used to represent p53 expression status, thus predict the p53-related chemotherapy sensitivity. In the clinic settings, we should set the cut-off value of SUVmax at around 5 to get the optimized sensitivity and specificity However, this is a more like bench study even if we used the clinical imaging technique. Using p53 as the biomarker for chemotherapy resistance in NSCLC is risky. Thus, cautions should be taken when using the SUVmax of FDG as an alternative or reliable marker for p53, not to mention the prognosis of NSCLC. To really apply the SUVmax of FDG in the clinic settings, more bench studies and clinic trials are needed. Further efforts are needed to reveal the underlying reasons for the inconsistency between the findings of ours and others, the study to fill the gap between our experimental findings and future clinical applications should also be considered.
Conclusions
In conclusion, the expressions of p53 and ERCC1 are associated with the SUVmax on FDG-PET in NSCLCs. Of the two markers, p53 expression is the primary predictor for the SUVmax. Based on our findings, FDG-PET might be a simple and good non-invasive method for predicting p53-related chemotherapy resistance in NSCLCs. But cautions should be taken when using this method in the clinical settings.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
XYD and YMG designed research; XYD, WW, JSW and SJ performed the research; XYD, WW and JGG analyzed data, XYD and WW wrote the paper. All authors read and approved the final manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Supplementary Material
Multiple stepwise regression analysis of the primary predictor for SUVmax.
Contributor Information
Xiao-Yi Duan, Email: duanxy@mail.xjtu.edu.cn.
Wen Wang, Email: wangwen@fmmu.edu.cn.
Jian-Sheng Wang, Email: wangjsh@mail.xjtu.edu.cn.
Jin Shang, Email: jinshang@mail.xjtu.edu.cn.
Jun-Gang Gao, Email: jungang@mail.xjtu.edu.cn.
You-Min Guo, Email: cjr.guoyoumin@vip.163.com.
Acknowledgements
This work was supported by the Natural Science Foundation of People’s Republic of China (No. 81171397).
Funding sources
This work was partly supported by Natural Science Foundation of China (No. 81171397).
References
- Siegel R, DeSantis C, Virgo K, Stein K, Mariotto A, Smith T, Cooper D, Gansler T, Lerro C, Fedewa S. et al. Cancer treatment and survivorship statistics, 2012. CA. 2012;62(4):220–241. doi: 10.3322/caac.21149. [DOI] [PubMed] [Google Scholar]
- Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clinic Proc. 2008;83(5):584–594. doi: 10.4065/83.5.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan K, Qian C, Zheng R. Prognostic significance of immunohistochemical Rac1 expression in survival in early operable non-small cell lung cancer. Med Sci Monit. 2009;15(11):BR313–BR319. [PubMed] [Google Scholar]
- Saisho S, Yasuda K, Maeda A, Yukawa T, Okita R, Hirami Y, Shimizu K, Nakata M. Post-recurrence survival of patients with non-small-cell lung cancer after curative resection with or without induction/adjuvant chemotherapy. Interact Cardiovasc Thorac Surg. 2013;16(2):166–172. doi: 10.1093/icvts/ivs450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth CM, Shepherd FA, Peng Y, Darling G, Li G, Kong W, Biagi JJ, Mackillop WJ. Time to adjuvant chemotherapy and survival in non-small cell lung cancer: a population-based study. Cancer. 2013;119(6):1243–1250. doi: 10.1002/cncr.27823. [DOI] [PubMed] [Google Scholar]
- Arriagada R, Bergman B, Dunant A, Le Chevalier T, Pignon JP, Vansteenkiste J. International Adjuvant Lung Cancer Trial Collaborative G. Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N Engl J Med. 2004;350(4):351–360. doi: 10.1056/NEJMoa031644. [DOI] [PubMed] [Google Scholar]
- Alam N, Shepherd FA, Winton T, Graham B, Johnson D, Livingston R, Rigas J, Whitehead M, Ding K, Seymour L. Compliance with post-operative adjuvant chemotherapy in non-small cell lung cancer. An analysis of National Cancer Institute of Canada and intergroup trial JBR.10 and a review of the literature. Lung Cancer. 2005;47(3):385–394. doi: 10.1016/j.lungcan.2004.08.016. [DOI] [PubMed] [Google Scholar]
- Stewart DJ, Chiritescu G, Dahrouge S, Banerjee S, Tomiak EM. Chemotherapy dose–response relationships in non-small cell lung cancer and implied resistance mechanisms. Cancer Treat Rev. 2007;33(2):101–137. doi: 10.1016/j.ctrv.2006.12.002. [DOI] [PubMed] [Google Scholar]
- d'Amato TA, Landreneau RJ, McKenna RJ, Santos RS, Parker RJ. Prevalence of in vitro extreme chemotherapy resistance in resected nonsmall-cell lung cancer. Ann Thorac Surg. 2006;81(2):440–446. doi: 10.1016/j.athoracsur.2005.08.037. discussion 446–447. [DOI] [PubMed] [Google Scholar]
- Pirker R, Pereira JR, von Pawel J, Krzakowski M, Ramlau R, Park K, de Marinis F, Eberhardt WE, Paz-Ares L, Storkel S. et al. EGFR expression as a predictor of survival for first-line chemotherapy plus cetuximab in patients with advanced non-small-cell lung cancer: analysis of data from the phase 3 FLEX study. Lancet Oncol. 2012;13(1):33–42. doi: 10.1016/S1470-2045(11)70318-7. [DOI] [PubMed] [Google Scholar]
- D’Amato TA, Landreneau RJ, Ricketts W, Huang W, Parker R, Mechetner E, Yu IR, Luketich JD. Chemotherapy resistance and oncogene expression in non-small cell lung cancer. J Thorac Cardiovasc Surg. 2007;133(2):352–363. doi: 10.1016/j.jtcvs.2006.10.019. [DOI] [PubMed] [Google Scholar]
- Simon GR, Ismail-Khan R, Bepler G. Nuclear excision repair-based personalized therapy for non-small cell lung cancer: from hypothesis to reality. Int J BiochemCell Biol. 2007;39(7–8):1318–1328. doi: 10.1016/j.biocel.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa H, Ikuma H, Asakura K, Uesaka K. Prognostic importance of standardized uptake value on F-18 fluorodeoxyglucose-positron emission tomography in biliary tract carcinoma. J Surg Oncol. 2009;100(6):494–499. doi: 10.1002/jso.21356. [DOI] [PubMed] [Google Scholar]
- Higashi K, Ueda Y, Sakurai A, Wang XM, Xu L, Murakami M, Seki H, Oguchi M, Taki S, Nambu Y. et al. Correlation of Glut-1 glucose transporter expression with. Eur J Nucl Med. 2000;27(12):1778–1785. doi: 10.1007/s002590000367. [DOI] [PubMed] [Google Scholar]
- Shimizu K, Hirami Y, Saisho S, Yukawa T, Maeda A, Yasuda K, Nakata M. Maximal standardized uptake value on FDG-PET is correlated with cyclooxygenase-2 expression in patients with lung adenocarcinoma. Ann Thorac Surg. 2012;93(2):398–403. doi: 10.1016/j.athoracsur.2011.10.033. [DOI] [PubMed] [Google Scholar]
- Watanabe K, Nomori H, Ohtsuka T, Naruke T, Ebihara A, Orikasa H, Yamazaki K, Uno K, Kobayashi T, Goya T. [F-18]Fluorodeoxyglucose positron emission tomography can predict pathological tumor stage and proliferative activity determined by Ki-67 in clinical stage IA lung adenocarcinomas. Jpn J Clin Oncol. 2006;36(7):403–409. doi: 10.1093/jjco/hyl043. [DOI] [PubMed] [Google Scholar]
- Kaira K, Oriuchi N, Shimizu K, Ishikita T, Higuchi T, Imai H, Yanagitani N, Sunaga N, Hisada T, Ishizuka T. et al. Correlation of angiogenesis with 18F-FMT and 18F-FDG uptake in non-small cell lung cancer. Cancer Sci. 2009;100(4):753–758. doi: 10.1111/j.1349-7006.2008.01077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs AR, Thunnissen FB. Histological typing of lung and pleural tumours: third edition. J Clin Pathol. 2001;54(7):498–499. doi: 10.1136/jcp.54.7.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin S. PET/CT for staging and monitoring non small cell lung cancer. Cancer Imaging. 2008;8(A):S27–S31. doi: 10.1102/1470-7330.2008.9006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koksal D, Demirag F, Bayiz H, Ozmen O, Tatci E, Berktas B, Aydogdu K, Yekeler E. The correlation of SUVmax with pathological characteristics of primary tumor and the value of Tumor/ Lymph node SUVmax ratio for predicting metastasis to lymph nodes in resected NSCLC patients. J Cardiothorac Surg. 2013;8:63. doi: 10.1186/1749-8090-8-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brabender J, Danenberg KD, Metzger R, Schneider PM, Park J, Salonga D, Holscher AH, Danenberg PV. Epidermal growth factor receptor and HER2-neu mRNA expression in non-small cell lung cancer Is correlated with survival. Clin Cancer Res. 2001;7(7):1850–1855. [PubMed] [Google Scholar]
- Taylor MD, Smith PW, Brix WK, Wick MR, Theodosakis N, Swenson BR, Kozower BD, Jones DR. Correlations between selected tumor markers and fluorodeoxyglucose maximal standardized uptake values in esophageal cancer. Eur J Cardiothorac Surg. 2009;35(4):699–705. doi: 10.1016/j.ejcts.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J, Liang X, Zhou X, Huang R, Chu Z, Zhan Q. ERCC1 expression as a prognostic and predictive factor in patients with non-small cell lung cancer: a meta-analysis. Mol Biol Rep. 2012;39(6):6933–6942. doi: 10.1007/s11033-012-1520-4. [DOI] [PubMed] [Google Scholar]
- Thottassery JV, Zambetti GP, Arimori K, Schuetz EG, Schuetz JD. p53-dependent regulation of MDR1 gene expression causes selective resistance to chemotherapeutic agents. Proc Natl Acad Sci USA. 1997;94(20):11037–11042. doi: 10.1073/pnas.94.20.11037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura H, Hirata T, Kitamura H, Nishikawa J. Correlation of the standardized uptake value in FDG-PET with the expression level of cell-cycle-related molecular biomarkers in resected non-small cell lung cancers. Ann Thorac Cardiovasc Surg. 2009;15(5):304–310. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Multiple stepwise regression analysis of the primary predictor for SUVmax.