Fig. 2.
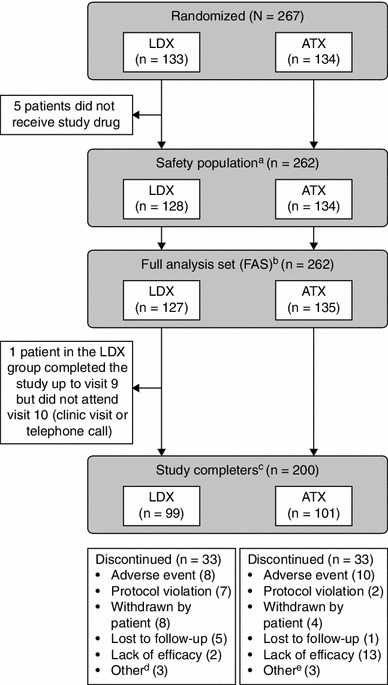
Patient disposition. aThe safety population included all patients who were randomized and received at least one dose of study drug. bThe FAS included all patients who were randomized and received at least one dose of study drug. One patient was randomized to ATX but, owing to a drug dispensing error, received LDX. Based on the intent-to-treat principle, this patient was included in the ATX treatment group in the FAS. cStudy completers were patients who completed visits 0–10 (visit 10 being a clinic visit or telephone call). dOther reasons for discontinuation among patients administered LDX were difficulty swallowing capsule (n = 1); early termination requested by the sponsor because of previous marijuana use (n = 1); and early termination requested by the sponsor because patient was unable to meet the study visit schedule (n = 1). eOther reasons for discontinuation among patients administered ATX were refusal to take medication (n = 1); patient relocation due to a family emergency (n = 1); and non-compliance (n = 1). ATX atomoxetine, FAS full analysis set, LDX lisdexamfetamine dimesylate
