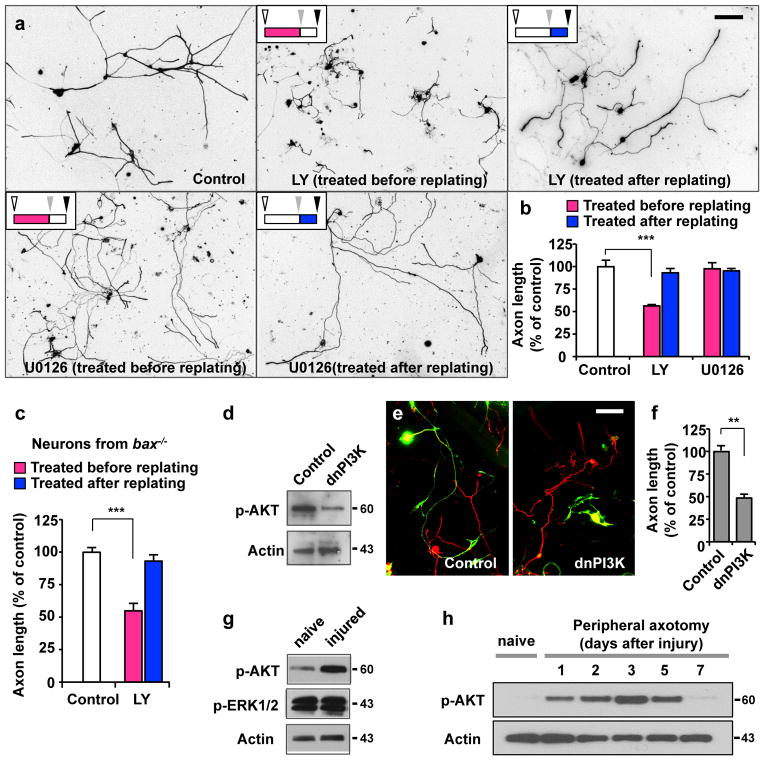
Figure 2. Activation of PI3K signalling is essential for augmentation of axon growth potential.
(a, b) Adult DRG neurons were cultured as depicted in Fig. 1a. Neurons were treated with LY294002 (LY, 10 μM), U0126 (20 μM), or vehicle control (DMSO) either before (red bars in b) or after replating (blue bars in b), as indicated. Regenerative axon growth was assessed by measuring axon length after replating. Representative images of replated neurons are shown in a. Quantification of axon length from three independent experiments is shown in b. Scale bar, 200 μm. Error bars represent s.e.m. *** p < 0.001, unpaired two-tailed student t test.
(c) Adult DRG neurons from bax−/− mice were cultured as depicted in Fig. 1a. Neurons were treated with LY294002 (LY, 10 μM) or vehicle control (DMSO) either before (red bar) or after replating (blue bar), as indicated. Regenerative axon growth was assessed by measuring axon length after replating. Quantification of axon length from three independent experiments is shown. Error bars represent s.e.m. *** p < 0.001, unpaired two-tailed student t test.
(d) Validation of the dominant-negative PI3K construct (dnPI3K). Representative immunoblots from 3-day-cultured DRG neurons transfected with either dnPI3K or a control vector (EGFP). (e, f) Dissociated adult DRG neurons were transfected in vitro with EGFP alone (control) or with dnPI3K (myc tagged). Neurons were cultured for 3 days, followed by replating to initiate axon growth anew. Neurons were fixed at 20 hr after replating and stained for neuronal tubulin βIII, TuJ1 (red) or the myc tag. Transfected neurons are shown in green (EGFP or myc positive neurons). Representative images of replated neurons are shown in e. Quantification of axon length from three independent experiments is shown in f. Scale bar, 100 μm. Error bars represent s.e.m. ** p < 0.01, unpaired two-tailed student t test.
(g) Representative immunoblots of phosphorylated Akt and ERK1/2 detected in DRGs from naïve or injured mice that were subjected to sciatic nerve transection. (h) Representative immunoblots of time-course analysis of AKT phosphorylation in DRGs in response to peripheral nerve injury. Original immunoblot images are shown in Supplementary Fig. S10.