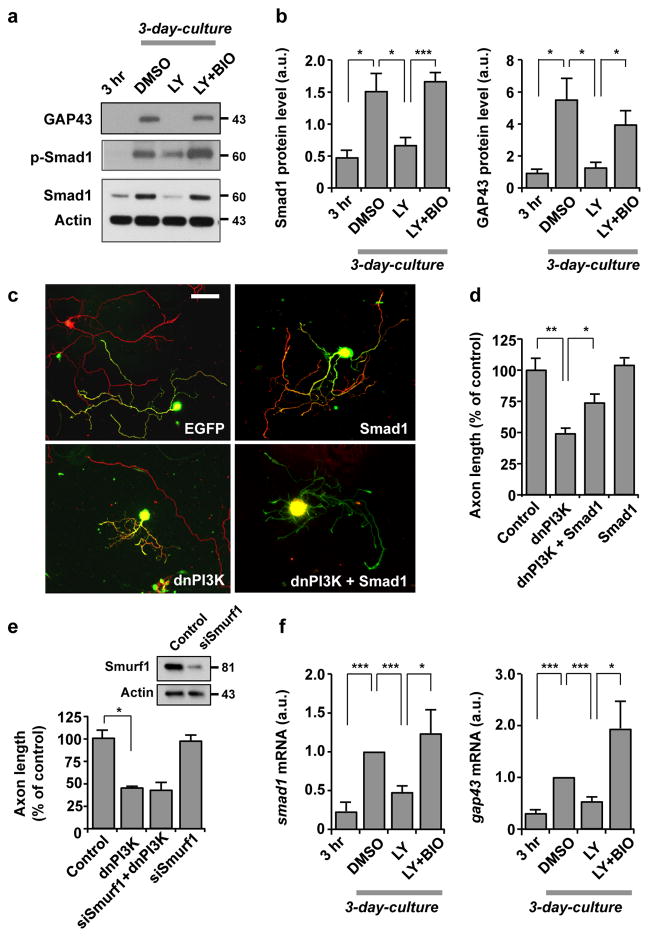
Figure 7. Induction of Smad1 downstream of the PI3K-GSK3 pathway is essential for augmentation of the transcription-dependent axon growth potential.
(a, b) Western blot analysis of DRG neurons cultured for 3 hr or 3 days to examine the levels of phospho-Smad1 (p-Smad1), Smad1 or GAP43. For the 3 day cultures, neurons were treated with DMSO, LY294002 (LY, 10 μM) and/or 6-bromoindirubin-3′-acetoxime (BIO, 500 nM). Representative images and quantification of Western blot analysis are shown in a and b. n=3, error bars represent s.e.m. * p < 0.05; *** p < 0.001, unpaired two-tailed student t test.
(c, d) Dissociated adult DRG neurons were transfected in vitro with EGFP, Smad1 (flag tagged) and/or dominant-negative PI3K (dnPI3K) (myc tagged). Neurons were cultured for 3 days and then replated for overnight culture. Neurons were then fixed and stained for neuronal tubulin, TuJ1, the flag tag, or the myc tag. Representative images of replated neurons are shown in c, and quantification of axon length from three independent experiments is shown in d. Scale bar, 100 μm. Error bars represent s.e.m. * p < 0.05; ** p < 0.001, unpaired two-tailed student t test.
(e) Dissociated adult DRG neurons were transfected with EGFP alone (control) or together with siRNAs against Smurf1 (siSmurf1) and/or dnPI3K, as indicated, and cultured for 3 days. Neurons were then replated and culture overnight. Quantification of axon length from three independent experiments is shown. Error bars represent s.e.m. * p < 0.05, unpaired two-tailed student t test. Efficacy of siSmurf1 was validated in adult DRG neurons (immunoblots in upper panel).
(f) Adult DRG neurons cultured for 3 hr or 3 days were subjected to quantitative real time polymerase chain reaction (qRT-PCR) to examine the mRNA level of smad1 or gap43. For the 3-day cultures, neurons were treated with DMSO, LY294002 (LY, 10 μM) and/or BIO (500 nM), as indicated. Values were normalized against the mRNA level of the DMSO-treated sample. n=3, error bars represent s.e.m. * p < 0.05; *** p < 0.001, unpaired two-tailed student t test. Original immunoblot images are shown in Supplementary Fig. S12.