Abstract
OBJECTIVE
To assess the magnitude of the dawn phenomenon and its impact on the total glucose exposure in type 2 diabetes.
RESEARCH DESIGN AND METHODS
A total of 248 noninsulin-treated persons with type 2 diabetes who underwent continuous glucose monitoring were divided into three groups selected by treatments: diet alone (n = 53); insulin sensitizers alone (n = 82); and insulin secretagogues alone or in combination with insulin sensitizers (n = 113). The dawn phenomenon (∂ glucose, mg/dL) was quantified by its absolute increment from nocturnal nadir to prebreakfast value. The participants were secondarily divided into two paired subsets after they had been separated by the presence/absence of a dawn phenomenon based on a threshold of 20 mg/dL and matched for glucose nadir. The impact of the dawn phenomenon was assessed on HbA1c and 24-h mean glucose.
RESULTS
The median of ∂ glucose (interquartile range) was 16.0 (0–31.5 mg/dL) in the 248 subjects, and no differences were observed across groups selected by HbA1c or treatments. In the overall population, the mean impacts on HbA1c and 24-h mean glucose were 4.3 ± 1.3 mmol/mol (0.39 ± 0.12%) and 12.4 ± 2.4 mg/dL, respectively. The mean impact on 24-h mean glucose was not statistically different between those on diet alone (16.7 ± 5.9 mg/dL) compared with the two subsets treated with oral hypoglycemic agents (11.2 ± 5.3 and 8.5 ± 7.5 mg/dL).
CONCLUSIONS
The impact of the dawn phenomenon on overall glycemic control in type 2 diabetes, as depicted by the HbA1c level, was ∼0.4% and not eliminated by any of the currently available armamentarium of oral antidiabetes agents.
The term “dawn phenomenon” was introduced by Schmidt et al. (1) in the early 1980s. Within a few years, it became obvious that this phenomenon corresponded to a spontaneous rise in plasma glucose and/or insulin requirements toward the end of the nocturnal period, in the absence of any intake of dietary carbohydrates (2). The dawn phenomenon was initially regarded as a causative factor for glucose instability, and although there are many uncertainties about its impact on overall glycemic control, these have never been adequately addressed. There are several potential reasons that can contribute to this deficiency. One of the main reasons resides in the fact that the definition is based on a dawn increment in glucose levels from the nocturnal nadir to the prebreakfast glucose value. As a consequence, the quantification of the dawn phenomenon requires accurate detection of the nocturnal nadir glucose concentration. Such determinations only became possible with the advent of continuous ambulatory interstitial glucose monitoring (CGM) (3,4), which permits the calculation of the absolute differences between the nocturnal nadirs and the prebreakfast glucose values. The current study was therefore designed using CGM to revisit the dawn phenomenon, which continues to remain a subject of debate (5–9). The first objective was to gain further insight into the assessment of its magnitude in a large group of persons with type 2 diabetes, using CGM on an ambulatory basis in real-life conditions on 2 consecutive days. In addition, we set out to explore the impact of the dawn phenomenon on the overall glucose exposure that was assessed both by the HbA1c levels and 24-h mean glucose concentrations calculated from the CGM using the average of 2 consecutive days to account for the interday fluctuations of the dawn phenomenon at an individual level. Should the current study be able to show that the presence or absence of the dawn phenomenon resulted in differences in glycemic control, this might incite health care providers to assess for and devise therapeutic strategies to avoid this phenomenon in an attempt to achieve better overall glycemic control in subjects with type 2 diabetes.
RESEARCH DESIGN AND METHODS
This study was conducted in a total of 248 persons with type 2 diabetes who were selected for final analysis after screening for eligibility among a total population of 292 subjects who underwent 3-day ambulatory CGM. Criteria of exclusion from the initial screened list of potential participants included those who had a HbA1c level ≥86 mmol/mol (≥10%) and/or had experienced a recent illness or been treated with steroids during the 3-month period preceding the investigation. In addition, exclusion criteria from the final analysis were unexpected disruption in the glucose monitoring or insufficient number of blood glucose tests for the calibration of the CGM (four tests were required daily for this purpose). Unacceptable calibration meant an accuracy criterion with a correlation coefficient of <0.79 (35 patients out of 292 [i.e., 12.0%]). Finally, we excluded all participants who reported at least one clinical hypoglycemic event over the test period in order to avoid any false interpretation of glucose rises in the early morning.
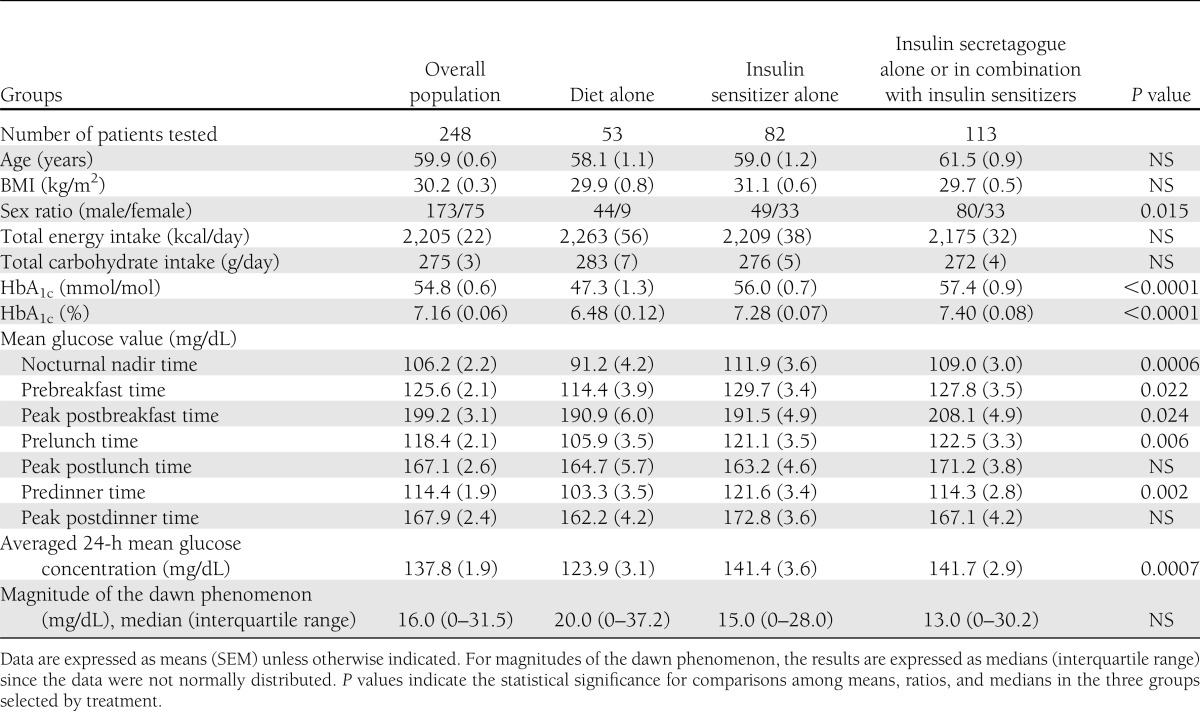
All participants were investigated from 2003 to 2011 at the outpatient clinics of either the University Hospital (Montpellier, France) for 198 subjects or the Diabetes Research Unit (Cardiff, U.K.) for 50 subjects with type 2 diabetes. All participants had been on a stable treatment regimen with dietary measures alone or with the addition of oral hypoglycemic agents (OHAs) for at least 3 months prior to CGM. Modalities of treatment were classified into three categories: 1) dietary measures alone (n = 53); 2) insulin sensitizers alone (metformin and/or thiazolidinediones) (n = 82); and 3) insulin secretagogues (sulfonylureas, glinides, or dipeptidyl peptidase-4 inhibitors) alone or in combination with an insulin sensitizer (n = 113). Dietary measures were based on a weight-maintaining diet with three main meals per day and with carbohydrates providing 50% of the total daily energy intake. Energy intake was assumed to be equal to total energy expenditure. The latter was determined by calculating the basal metabolic rate from the Schofield equations (10) and then by multiplying this result by 1.35, a coefficient that corresponds to a sedentary lifestyle with a poor physical activity in most of our patients. A daily ratio of 1:2:2 was recommended for calorie and carbohydrate distribution among breakfast, lunch, and dinner, respectively. The expected mean daily energy and carbohydrate intakes as estimated from this calculation are given in Table 1. At the beginning and end of each 3-day study period, dietary recommendations were carefully repeated and validated by trained dietitians, and instructions were given to patients for following their routine low-level physical activity and avoiding any vigorous exercise. All of the investigations were performed in routine diabetes outpatient clinics and were in compliance with the Helsinki Declaration (11). The study was conducted after each subject had given an oral informed consent in accordance with European directives that require no approval from an ethics committee for the noninterventional design as described herein (12).
Table 1.
Main clinical characteristics and treatments of patients in the overall population and in the different groups individualized according to whether patients were treated with diet alone, insulin sensitizers alone, and insulin secretagogues alone or in combination with insulin sensitizers
Clinical investigations and laboratory determinations
All participants underwent ambulatory CGM for 3 consecutive days using the same technology from 2003 to 2011 (i.e., a second-generation MiniMed system [Medtronic, Northridge, CA]) following training in the use of the device. The sensor was inserted on day 0 and removed midmorning on day 3. Insertion of CGM was made either on Monday or Tuesday and removal on Thursday or Friday in order to avoid any overlap over weekend days. All calculations were derived from data obtained over a 48-h period during days 1 and 2 in order to avoid any bias due to both insertion and removal of the sensor. Sustained chronic hyperglycemia (ambient hyperglycemia) was assessed on study day 0 by HbA1c levels that were determined using a high-performance liquid chromatography assay (13–15). The intra- and interassay coefficients of variation were <2% with a nondiabetic reference range of 16.7–32.1 mmol/mol (3.7–5.1%) HbA1c.
Analysis of the data obtained from the CGM
The CGM was used for identifying and quantifying both the nocturnal nadir, premeal and postmeal peak glucose levels. Prebreakfast time points were determined in accordance with the data recorded by each participant in his/her own log book. All readings of CGM were centralized in Montpellier and concomitantly made by two investigators using the same methodology for analysis and interpretation of the data of the glycemic profiles from 2003 to 2011. All measurements provided by CGM are presented having averaged the data recorded on study days 1 and 2. This strategy was used because a prestudy analysis of the glucose profiles had indicated that the dawn phenomenon was subject to large interday fluctuations at an individual level. In the 248 subjects included in the present analysis, the comparison of the data recorded on study days 1 and 2 showed that the median of interday difference from nocturnal glucose nadir to prebreakfast glucose value was equal to 15.0 mg/dL (interquartile range 0–21.0 mg/dL). Given this observation and in order to attenuate the day-to-day fluctuations of the data of the glucose profiles including those of the dawn phenomenon, we therefore decided to conduct the final analysis of the glucose profiles after all data had been averaged on the 2 consecutive days of the glucose monitoring.
The dawn phenomenon (i.e., the spontaneous glucose rise during early morning hours) was quantified by subtracting the glucose nadir from the glucose value observed just before the beginning of breakfast. Even though measurements of glucose in the interstitial fluid from CGM readings are lower than venous plasma glucose concentrations (16), it should be noted that in the current study, the dawn phenomenon was assessed from differences between interstitial values. The upward glycemic variation during the night was only considered a dawn phenomenon if its magnitude was above the stochastic spontaneous glucose fluctuations during the early morning period after an overnight fast. According to this remark, a threshold of 20 mg/dL was chosen because it appears more tenable than others that were previously used (7,8). This choice was based on three main principles. First, a value of 20 mg/dL approximates to the 95% CI of total intra- and interassay variability applied to a true fasting glucose concentration of 126 mg/dL (17). This value is comparable to those observed at prebreakfast times in the overall population and in the different groups of the current study (Table 1). Second, this value of 20 mg/dL is nearest to that recently observed for the mean magnitude of the dawn glucose rise in type 2 diabetes (9). Third, as indicated above and according to the calculations that were made by comparing the dawn glucose rises on study days 1 and 2, the median of interday difference was 15 mg/dL.
Assessment of the impact of the dawn phenomenon on the overall glucose exposure
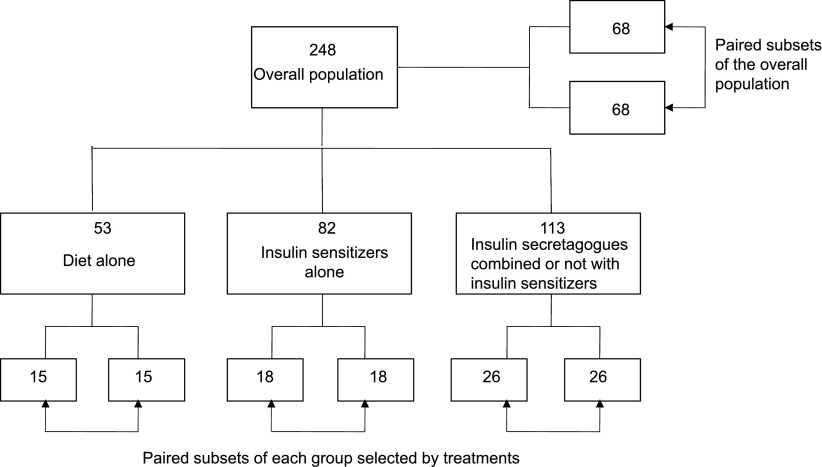
In order to assess the impact of the dawn phenomenon on the overall glucose exposure, the subjects of the total investigated population (n = 248) and of the different groups selected by treatments were divided according to whether they exhibited or not a dawn glucose increase >20 mg/dL. All separated patients were then matched for the nocturnal glucose nadir that was taken as reference for the calculation of the dawn glucose increment. The latter procedure was rendered necessary in order to align pairs of patients on the same glucose reference and to avoid any bias when comparing the data between individuals who exhibited a dawn phenomenon and those who did not. Using these criteria, the numbers of subjects remaining in the different paired subsets after selection for the presence or absence of the dawn phenomenon and after matching for nocturnal glucose nadir are indicated in Fig. 1. The impact of the dawn phenomenon on the overall glucose exposure was quantified by comparing the mean differences in the averaged 24-h mean glucose concentrations and HbA1c levels between the paired subsets of subjects who were matched for glucose nadir and separated according to the presence or absence of dawn phenomenon.
Figure 1.
Steps used for analyzing the impact of the dawn phenomenon in the overall population and in the different groups selected by treatments. In the different groups including the overall population, the patients were secondarily separated into two paired subsets after selection for presence/absence of a dawn phenomenon (threshold set at 20 mg/dL) and after matching for nocturnal glucose nadir. Number of patients in each group and subset are indicated inside each square.
Statistical analysis
Results are given as mean ± SEM or median with interquartile ranges as appropriate. For instance, the median was used for analyzing the magnitude of the dawn phenomenon (∂ glucose) since its distribution was asymmetrical and skewed toward low values. The Kruskal-Wallis test was used for multiple median comparisons of asymmetrical distributed data. The ANOVA test or the Student t test were used for comparison of means of normally distributed data, as appropriate. Analysis were performed with the STATVIEW statistical package for Macintosh (programs from SAS Institute, Cary, NC).
RESULTS
Clinical and laboratory data of the entire study population (n = 248) and for the three different groups selected by treatment are included in Table 1, after exclusion of the patients who did not fulfill the selection criteria. Age and BMI, but not sex ratio, were similar in all groups. The mean maximum glucose concentrations of the circadian glycemic profiles were observed after breakfast during the morning period. In all groups, the prebreakfast mean glucose concentrations (i.e., the value after the overnight fast) were significantly higher (P < 0.0001) than the nadir values observed during the nocturnal periods.
Magnitude of the dawn phenomenon
The absolute median glucose increments (∂ glucose, mg/dL) from nocturnal nadir to prebreakfast value did not statistically differ (P = 0.37) when the different groups selected by categories of treatment were compared (Table 1). Slight but not significant differences (P = 0.14) were observed across groups of subjects individualized according to their HbA1c levels: median ∂ glucose (interquartile range) 11.0 (0–26.8 mg/dL), 19.5 (0–33.0 mg/dL), and 18.0 (0–39.5 mg/dL) according to whether the subjects had HbA1c levels <53 mmol/mol (<7%; n = 111), between 53 and 62.9 mmol/mol (7–7.9%; n = 90), and ≥64 mmol/mol (≥8%; n = 47), respectively.
Impact of the dawn phenomenon on the total glucose exposure
As indicated in Table 2, when the glucose threshold of the dawn phenomenon was set at 20 mg/dL, both the HbA1c levels and the averaged 24-h mean glucose values in the paired subsets of subjects matched for glucose nadir in the overall population (n = 136) were statistically greater (P = 0.007 and P = 0.0009, respectively) in those subjects who exhibited the dawn phenomenon compared with those who did not. The mean differences between the two paired subsets were 4.3 ± 1.3 mmol/mol for HbA1c and 12.4 ± 2.4 mg/dL for averaged 24-h mean intracutaneous glucose concentrations. An illustration of these results is given by the differences of the mean glycemic profiles obtained in the two paired subsets of participants included in the overall population after matching for nocturnal nadirs (Fig. 2A).
Table 2.
Impact of the dawn phenomenon on the total glucose exposure
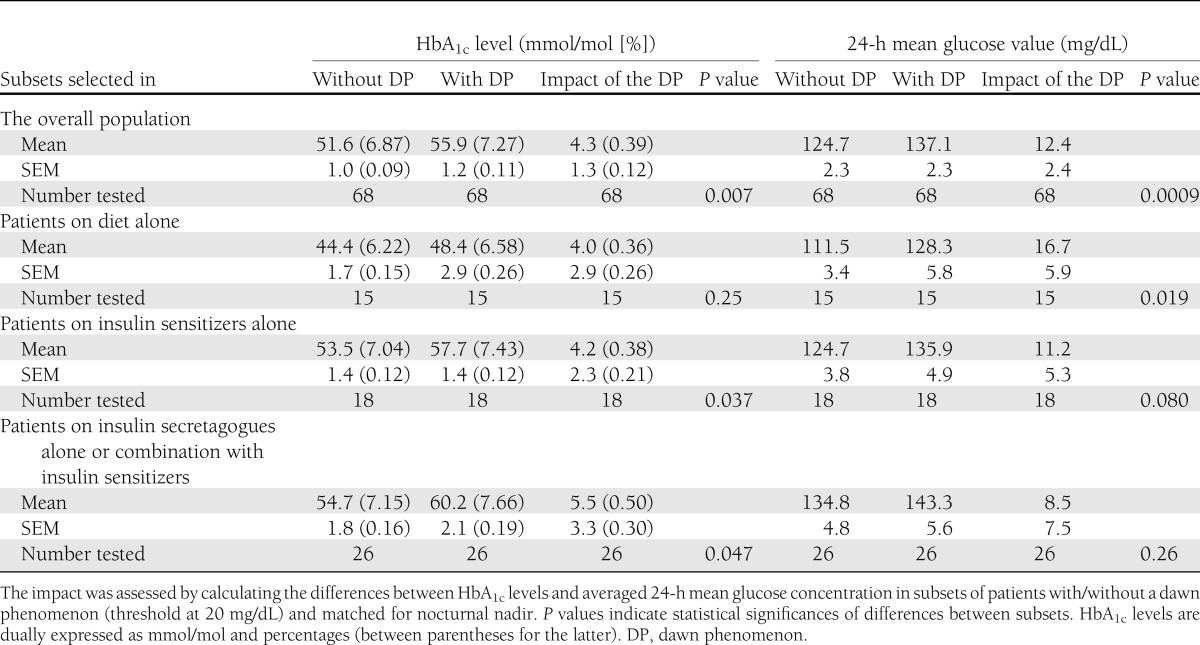
Figure 2.
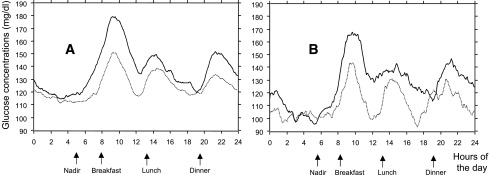
Mean averaged 24-h continuous glucose profiles in the two paired subsets of patients derived from either the overall population (A) (n = 68 in each subset) or the group treated with diet alone (B) (n = 15 in each subset). The subsets were selected after patients had been separated for the presence/absence of a dawn phenomenon. Solid curves, subsets of patients with the presence of a dawn phenomenon; dotted curves, subsets of patients with the absence of a dawn phenomenon.
Considering the participants when selected by treatments, the differences were found to be less significant than in the overall population (Table 2). In the two paired subsets treated with diet alone, those who had a dawn glucose rise >20 mg/dL exhibited greater averaged 24-h mean glucose concentrations (P = 0.019) than the subjects whose glucose levels remained below this threshold (Table 2). By contrast, HbA1c levels were not significantly different. An illustration of the results of the mean glycemic profiles obtained in the two paired subsets of participants who were treated with dietary measures alone and matched for nocturnal nadirs is given in Fig. 2B. In the two remaining paired subsets (i.e., in the patients treated with OHAs), the HbA1c levels differed significantly, while the averaged 24-h mean glucose concentrations did not (Table 2). The highest difference of dawn impacts on 24-h mean glucose value between patients with/without a dawn phenomenon was observed in the subset of patients who were treated with diet alone: 16.7 ± 5.9 mg/dL (Table 2). The impacts of the dawn phenomenon were less, although not significantly lower, in the pharmacologically treated subjects: 11.2 ± 5.3 mg/dL in persons on insulin sensitizers alone and 8.5 ± 7.5 mg/dL in those on insulin secretagogues alone or in combination with insulin sensitizers.
CONCLUSIONS
The present results, which were analyzed after a rigorous validation of glucose monitoring, indicate that in non–insulin-treated persons with type 2 diabetes, the median magnitude of the dawn glucose increase is ∼16 mg/dL and relatively stable across the groups of subjects selected either by categories of treatment or HbA1c levels. In the population considered as a whole and in the different groups selected by treatments, the highest peak glucose value of the circadian glucose profile was observed during the postbreakfast period. This observation is in agreement with previous reports (17,18). These excessive postbreakfast glucose excursions correspond to the so-called “extended dawn phenomenon,” which is characterized by the fact that the glucose tolerance is worse in the early morning than at any other time of the day (19). The dawn and extended dawn phenomena behave conjointly since they depend on an increase in both hepatic glucose output/neoglucogenesis and peripheral insulin resistance in the early morning period (20,21). Consequently, it is important first to assess whether the dawn phenomenon exerts a remnant effect on the glycemic profile and further to quantify this impact on the overall glucose exposure which was the main purpose of this study. The comparison of the subsets matched for the nocturnal glucose nadir and separated by the presence/absence of a dawn phenomenon showed that the differences in HbA1c levels and mean glucose concentrations were ∼4 mmol/mol (0.4%) and 12 mg/dL, respectively. However, it should be noted that discrepancies in statistical significances were observed in the different groups. In the subjects treated with diet alone, the differences were only significant for the mean glucose concentrations and not for HbA1c, while the reverse was observed in the patients on OHAs. Such discrepancies could be due to the reduction in sample size when participants were divided into subsets. Also, it is always difficult to extrapolate to prolonged periods of time (∼3 months for the HbA1c [13–15]) the effect of the presence or absence of the dawn phenomenon, as its assessment on 2 consecutive days is not necessarily a reflection of what happens during longer periods of time. The dawn glucose rise from nocturnal nadir to prebreakfast time is subject to large day-to-day fluctuations, supported by the fact that in the current study, the median of between-day difference in the magnitude of the dawn phenomenon is as high as 15.0 mg/dL. Therefore, averaging the dawn phenomenon on 2 consecutive days and assessing its impact on the averaged 2-day mean glucose concentrations and HbA1c levels is an approach that was designed for attenuating its interday variability. Extending the CGM for longer periods risked the loss of both reliability of the glucose sensor and acceptance of the monitoring device by the participants. Our findings are supported by the fact that the impact of the dawn phenomenon in the overall population (12 mg/dL on the mean glucose concentration and 4 mmol/mol [i.e., 0.4% on HbA1c]) is in broad agreement with the relationship that was previously established by Nathan et al. (22) in the A1C-Derived Average Glucose study (i.e., any increment of 29 mg/dL in mean glucose concentration corresponds to a 1% increment in HbA1c).
In conclusion, the remnant effect of the dawn phenomenon on the overall diabetic control is ∼4 mmol/mol (i.e., ∼0.4% HbA1c). Taking as reference the paired subsets of participants treated with diet alone, it appears that the dawn phenomenon is already present in those who are free of any pharmacological treatment with antidiabetes agents. Even though its impact seems to be slightly lower in individuals on OHAs, our results indicate that the dawn phenomenon has a remnant impact in subjects treated with our current oral antidiabetes armamentarium (23).
Acknowledgments
This study was supported by academic funds provided by the Universities of Montpellier I and Cardiff.
No potential conflicts of interest relevant to this article were reported.
L.M. contributed to the study design, participated in patient clinical care, analyzed data, and wrote, reviewed, and edited the manuscript. C.C. analyzed data and reviewed and edited the manuscript. S.D. contributed to the study design and reviewed the manuscript. D.O. contributed to the study design, participated in patient clinical care, and reviewed the manuscript. L.M. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
See accompanying commentary, p. 3860.
References
- 1.Schmidt MI, Hadji-Georgopoulos A, Rendell M, Margolis S, Kowarski A. The dawn phenomenon, an early morning glucose rise: implications for diabetic intraday blood glucose variation. Diabetes Care 1981;4:579–585 [DOI] [PubMed] [Google Scholar]
- 2.Schmidt MI, Lin QX, Gwynne JT, Jacobs S. Fasting early morning rise in peripheral insulin: evidence of the dawn phenomenon in nondiabetes. Diabetes Care 1984;7:32–35 [DOI] [PubMed] [Google Scholar]
- 3.Buckingham B, Black J, Wilson DM. Continuous glucose monitoring. Curr Opin Endocrinol Diabetes 2005;12:273–279 [DOI] [PubMed] [Google Scholar]
- 4.Skyler JS. Continuous glucose monitoring: an overview of its development. Diabetes Technol Ther 2009;11(Suppl. 1):S5–S10 [DOI] [PubMed] [Google Scholar]
- 5.Bolli GB, Gerich JE. The “dawn phenomenon”—a common occurrence in both non-insulin-dependent and insulin-dependent diabetes mellitus. N Engl J Med 1984;310:746–750 [DOI] [PubMed] [Google Scholar]
- 6.Atiea JA, Luzio S, Owens DR. The dawn phenomenon and diabetes control in treated NIDDM and IDDM patients. Diabetes Res Clin Pract 1992;16:183–190 [DOI] [PubMed] [Google Scholar]
- 7.Carroll MF, Hardy KJ, Burge MR, Schade DS. Frequency of the dawn phenomenon in type 2 diabetes: implications for diabetes therapy. Diabetes Technol Ther 2002;4:595–605 [DOI] [PubMed] [Google Scholar]
- 8.Carroll MF, Schade DS. The dawn phenomenon revisited: implications for diabetes therapy. Endocr Pract 2005;11:55–64 [DOI] [PubMed] [Google Scholar]
- 9.Monnier L, Colette C, Sardinoux M, Baptista G, Regnier-Zerbib A, Owens D. Frequency and severity of the dawn phenomenon in type 2 diabetes: relationship to age. Diabetes Care 2012;35:2597–2599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schofield WN, Schofield C, James WPT. Basal metabolic rate—review and prediction, together with an annotated bibliography of source material. Hum Nutr Clin Nutr 1985;39C(Suppl. 1):1–96 [PubMed] [Google Scholar]
- 11.World Medical Association Declaration of Helsinki. Recommendations guiding physicians in biomedical research involving human subjects. JAMA 1997;277:925–926 [PubMed] [Google Scholar]
- 12.Directive 2001/20/CE of the European Parliament and of the council of 4 April 2001 on the approximation of the laws, regulations and administrative provisions of the Member States relating to the implementation of good clinical trials on medicinal products for human use. Off J Eur Communities 2001;L121:33–44 [PubMed] [Google Scholar]
- 13.Gorus F, Mathieu C, Gerlo E. How should HbA1c measurements be reported? Diabetologia 2006;49:7–10 [DOI] [PubMed] [Google Scholar]
- 14.Sacks DB, Bruns DE, Goldstein DE, MacLaren NK, McDonald JM, Parrott M. Position statement executive summary: guidelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus (position statement). Diabetes Care 2002;25:750–78621617111 [Google Scholar]
- 15.Sacks DB. Measurement of hemoglobin A1c: a new twist on the path to harmony. Diabetes Care 2012;35:2674–2680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Monsod TP, Flanagan DE, Rife F, et al. Do sensor glucose levels accurately predict plasma glucose concentrations during hypoglycemia and hyperinsulinemia? Diabetes Care 2002;25:889–893 [DOI] [PubMed] [Google Scholar]
- 17.Ollerton RL, Playle R, Ahmed K, Dunstan FD, Luzio SD, Owens DR. Day-to-day variability of fasting plasma glucose in newly diagnosed type 2 diabetic subjects. Diabetes Care 1999;22:394–398 [DOI] [PubMed] [Google Scholar]
- 18.Monnier L, Colette C, Rabasa-Lhoret R, et al. Morning hyperglycemic excursions: a constant failure in the metabolic control of non-insulin-using patients with type 2 diabetes. Diabetes Care 2002;25:737–741 [DOI] [PubMed] [Google Scholar]
- 19.Monnier L, Colette C, Dunseath GJ, Owens DR. The loss of postprandial glycemic control precedes stepwise deterioration of fasting with worsening diabetes. Diabetes Care 2007;30:263–269 [DOI] [PubMed] [Google Scholar]
- 20.Boden G, Chen X, Urbain JL. Evidence for a circadian rhythm of insulin sensitivity in patients with NIDDM caused by cyclic changes in hepatic glucose production. Diabetes 1996;45:1044–1050 [DOI] [PubMed] [Google Scholar]
- 21.Perriello G, Pampanelli S, Del Sindaco P, et al. Evidence of increased systemic glucose production and gluconeogenesis in an early stage of NIDDM. Diabetes 1997;46:1010–1016 [DOI] [PubMed] [Google Scholar]
- 22.Nathan DM, Kuenen J, Borg R, Zheng H, Schoenfeld D, Heine RJ, A1c-Derived Average Glucose (ADAG) Study Group Translating the A1C assay into estimated average glucose values. Diabetes Care 2008;31:1473–1478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycaemia in type 2 diabetes: a patient-centered approach. Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 2012;55:1577–1596 [DOI] [PubMed] [Google Scholar]