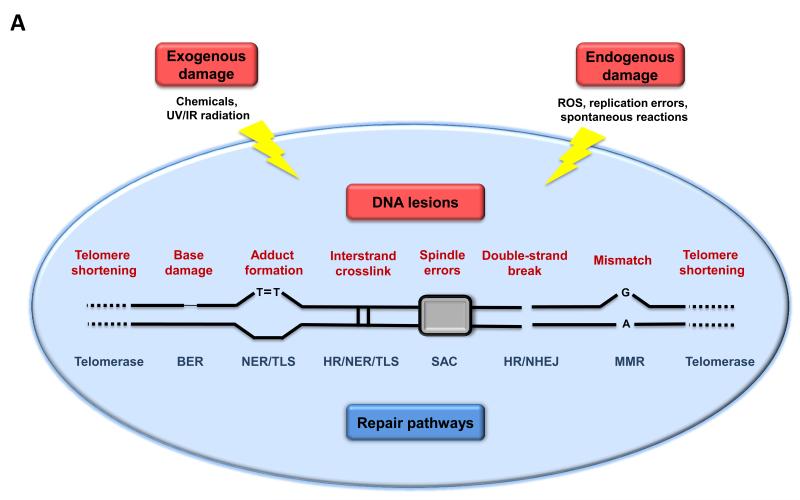
Figure 2. Genomic and Epigenomic Alterations.
A) Genomic instability and telomere attrition. Endogenous or exogenous agents can stimulate a variety of DNA lesions that are schematically represented on one single chromosome. Such lesions can by repaired by a variety of mechanisms. Excessive DNA damage or insufficient DNA repair favors the aging process. Note that both nuclear DNA and mitochondrial DNA (not represented here) are subjected to age-associated genomic alterations. BER, base excision repair; HR, homologous recombination; NER, nucleotide excision repair; NHEJ, non-homologous end joining; MMR, mismatch repair; ROS, reactive oxygen species; TLS, translesion synthesis; SAC, spindle assembly checkpoint.
B) Epigenetic alterations. Alterations in the acetylation and methylation of DNA or histones, as well as of other chromatin-associated proteins, can induce epigenetic changes that contribute to the aging process.