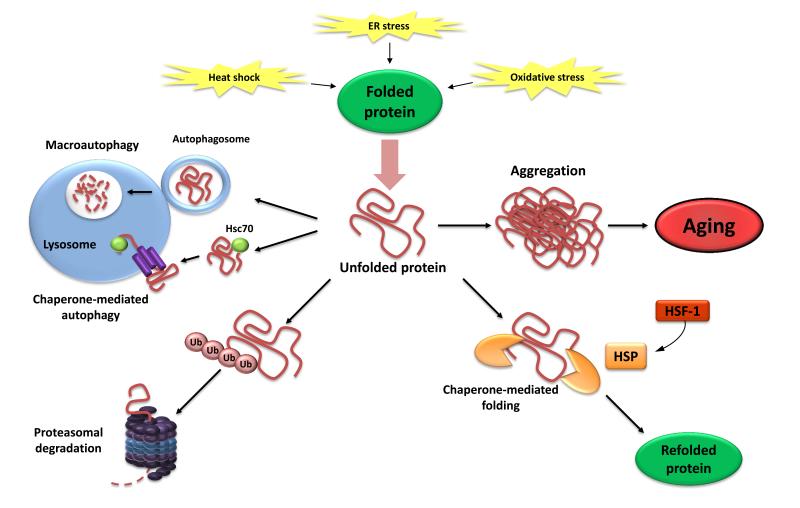
Figure 3. Loss of Proteostasis.
Endogenous and exogenous stress causes the unfolding of proteins (or impairs proper folding during protein synthesis). Unfolded proteins are usually refolded by heat-shock proteins (HSP) or targeted to destruction by the ubiquitin-proteasome or lysosomal (autophagic) pathways. The autophagic pathways include recognition of unfolded proteins by the chaperone Hsc70 and their subsequent import into lysosomes (chaperone-mediated autophagy) or sequestration of damaged proteins and organelles in autophagosomes that later fuse with lysosomes (macroautophagy). Failure to refold or degrade unfolded proteins can lead to their accumulation and aggregation, resulting in proteotoxic effects.