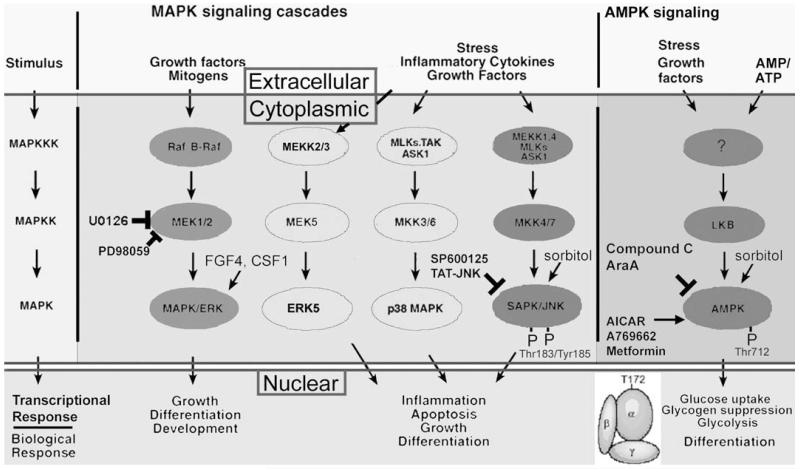
FIGURE 4.
Stress and the Kinome. Stress may influence a substantial fraction of the 510 enzymes shared by the human and mouse protein kinase kinome, but several members of the MAPK family (SAPK/JNK, p38MAPK, and ERK5) and the two genes encoding the alpha catalytic subunit of AMPK are important in numerous examples of the stress response of oocytes, preimplantation embryos, and TSC. The AMPK and MAPK superfamilies are similar in being activated by a number of stimuli including many of those listed in Figure 2, but AMPK has been characterized as an energy cellular sensor, primarily being rapidly activated by increases in the cellular AMP/ATP ratio that indicates cellular energy depletion. SAPK/JNK and AMPK are blocked by chemical and polypeptide inhibitors (SP600125 and TAT-JNK/LJNKl1, and Compound C and AraA, respectively). Activation of endogenous SAPK/JNK and AMPK is completely correlated with phosphorylation by upstream kinases of Thr183/Tyr185 and Thr 172, respectively. The amplifying cascade of protein kinases results in cytoplasmic effects such as metabolic control through management of rate limiting enzymes in anabolic and catabolic processes, and by nuclear control of transcription factors that mediate more profound and far-reaching changes in cell potency and proliferation states.