Abstract
We have shown [Mesa-Tejada, R., Keydar, I., Ramanarayanan, M., Ohno, T., Fenoglio, C. & Spiegelman, S. (1978) Proc. Natl. Acad. Sci. USA 75, 1529--1533] that an antigen immunologically related to gp52, a 52,000-dalton glycoprotein of the mouse mammary tumor virus, can be identified in sections of human breast cancer by means of an indirect immunoperoxidase technique. The specificity of the reaction was established by absorption experiments which revealed that only purified gp52, or material containing it, served to eliminate the IgG molecules responsible for the immunohistochemical reaction in the human breast tumors. We show here that the cross-reactivity between the human and murine tumor antigens is due to the polypeptide rather than the polysaccharide components of gp.52. Sugar-free gp52 prepared by deglycosylation with a mixture of glycosidases was as fully effective as the intact gp52 in removing from anti-MMTV the IgG responsible for the reaction with the human tumor antigen. In contrast, the isolated polysaccharide of gp52 was unable to exert blocking activity.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axel R., Gulati S. C., Spiegelman S. Particles containing RNA-instructed DNA polymerase and virus-related RNA in human breast cancers. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3133–3137. doi: 10.1073/pnas.69.11.3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axel R., Schlom J., Spiegelman S. Presence in human breast cancer of RNA homologous to mouse mammary tumour virus RNA. Nature. 1972 Jan 7;235(5332):32–36. doi: 10.1038/235032a0. [DOI] [PubMed] [Google Scholar]
- Black M. M., Zachrau R. E., Dion A. S., Shore B., Fine D. L., Leis H. P., Jr, Williams C. J. Cellular hypersensitivity of gp55 of RIII-murine mammary tumor virus and gp55-like protein of human breast cancers. Cancer Res. 1976 Nov;36(11 Pt 1):4137–4142. [PubMed] [Google Scholar]
- Black M. M., Zachrau R. E., Shore B., Leis H. P., Jr Biological considerations of tumor-specific and virus-associated antigens of human breast cancers. Cancer Res. 1976 Feb;36(2 Pt 2):769–774. [PubMed] [Google Scholar]
- Bolton A. E., Hunter W. M. The labelling of proteins to high specific radioactivities by conjugation to a 125I-containing acylating agent. Biochem J. 1973 Jul;133(3):529–539. doi: 10.1042/bj1330529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen J. M., Dmochowski L., Miller M. F., Priori E. S., Seman G., Dodson M. L., Maruyama K. Implications of humoral antibody in mice and humans to breast tumor and mouse mammary tumor virus-associated antigens. Cancer Res. 1976 Feb;36(2 Pt 2):759–764. [PubMed] [Google Scholar]
- Charney J., Moore D. H. Neutralization of murine mammary tumour virus by sera of women with breast cancer. Nature. 1971 Feb 26;229(5287):627–628. doi: 10.1038/229627a0. [DOI] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Holder W. D., Jr, Peer G. W., Bolognesi D. P., Wells S. A., Jr Antibody reacting with mouse mammary tumor virus in serum of breast carcinoma patients. Surg Forum. 1976;27(62):102–104. [PubMed] [Google Scholar]
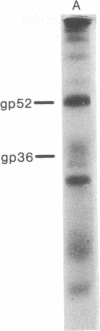
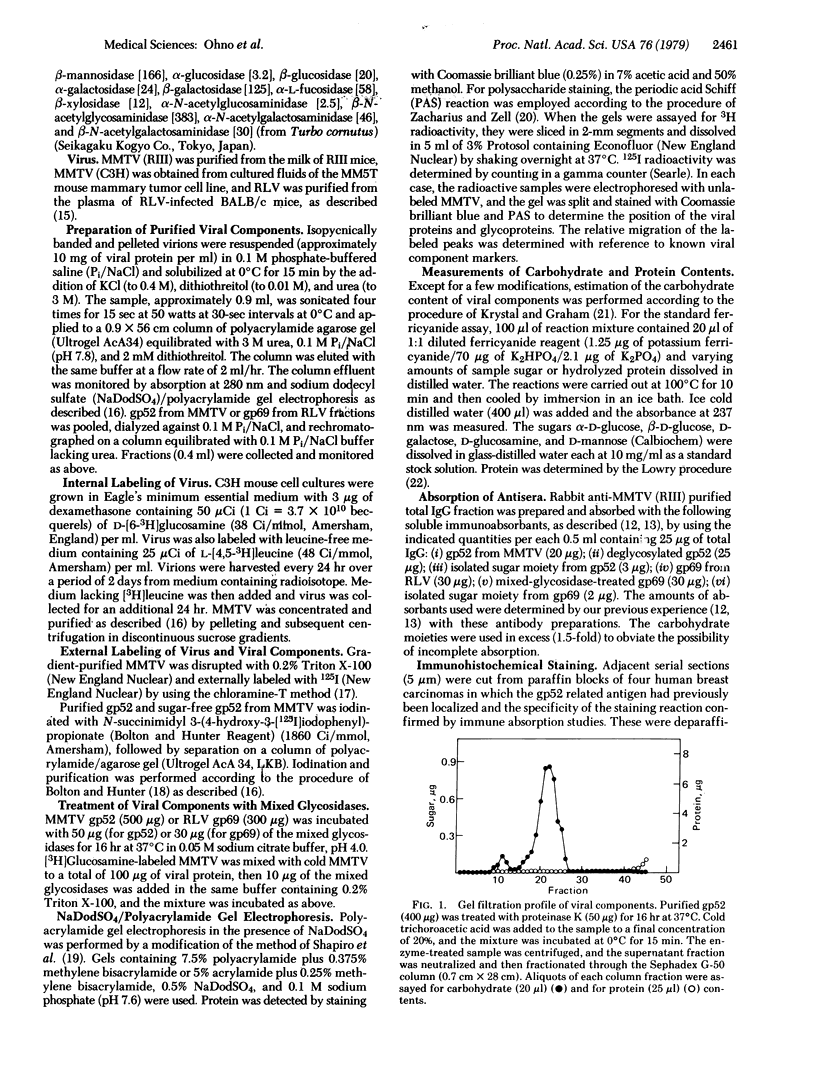
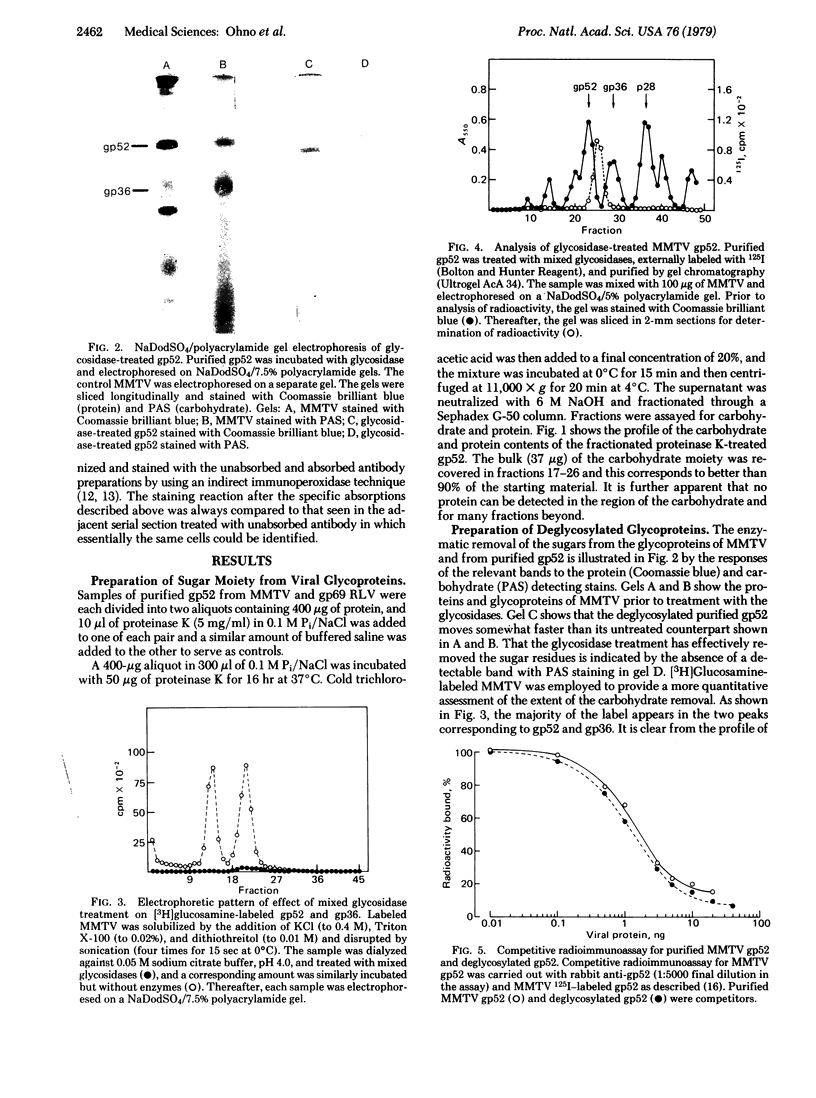
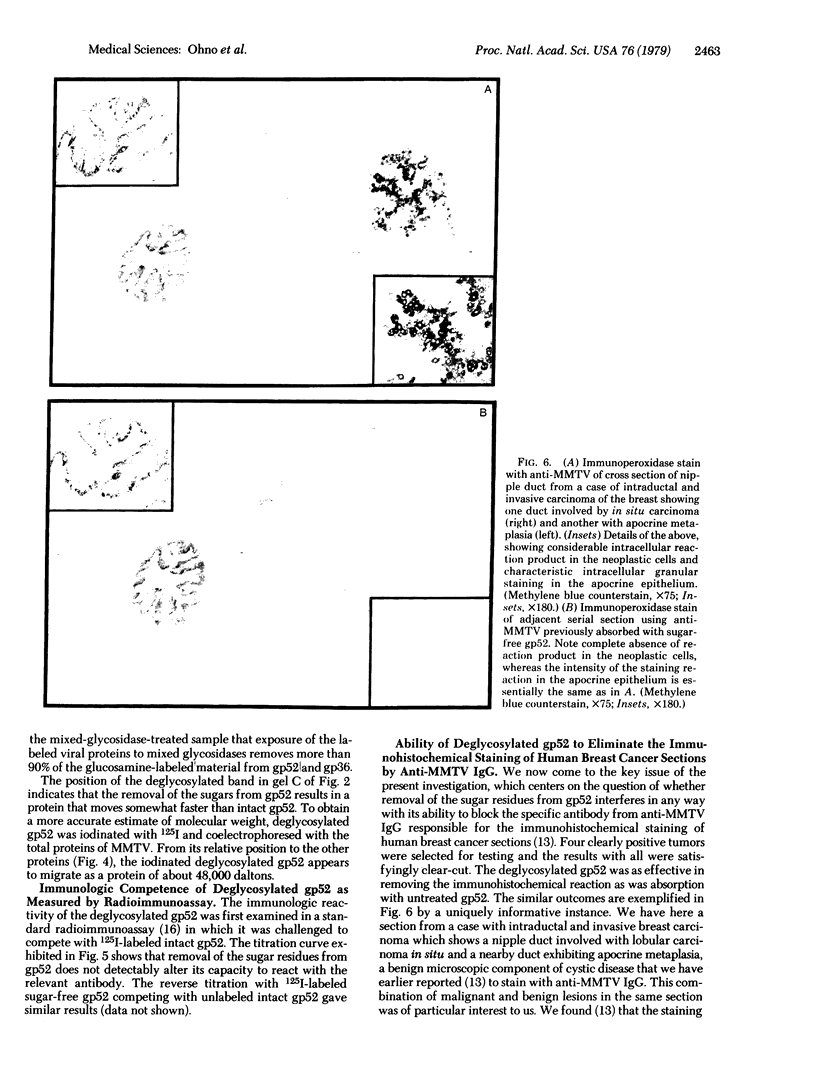
- Keydar I., Mesa-Tejada R., Ramanarayanan M., Ohno T., Fenoglio C., Hu R., Spiegelman S. Detection of viral proteins in mouse mammary tumors by immunoperoxidase staining of paraffin sections. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1524–1528. doi: 10.1073/pnas.75.3.1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystal G., Graham A. F. A sensitive method for estimating the carbohydrate content of glycoproteins. Anal Biochem. 1976 Feb;70(2):336–345. doi: 10.1016/0003-2697(76)90454-1. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Menzel R., Michaelis D., Neumann I., Schultz B., Wilke W., Wulfert P., Michael R., Bibergeil H. Remission phase in newly diagnosed insulin treated juvenile diabetes: behaviour of glucose, FFA, HGH and total-IRI during combined glucose arginine infusion; development of antibodies against exogenous insulin. Endokrinologie. 1973 Aug;62(1):100–106. [PubMed] [Google Scholar]
- Mesa-Tejada R., Keydar I., Ramanarayanan M., Ohno T., Fenoglio C., Spiegelman S. Detection in human breast carcinomas of an antigen immunologically related to a group-specific antigen of mouse mammary tumor virus. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1529–1533. doi: 10.1073/pnas.75.3.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller M., Zotter S., Grossmann H., Kemmer C. Immunologische Kreuzreaktion zwischen Brustkrebs und Mastopathie des Menschen sowie virusproduzierenden Mammakarzinomen der Maus. Arch Geschwulstforsch. 1972;40(4):285–299. [PubMed] [Google Scholar]
- Ritzi E., Baldi A., Spiegelman S. The purification of a gs antigen of the murine mammary tumor virus and its quantitation by radioimmunoassay. Virology. 1976 Nov;75(1):188–197. doi: 10.1016/0042-6822(76)90017-9. [DOI] [PubMed] [Google Scholar]
- Shapiro A. L., Viñuela E., Maizel J. V., Jr Molecular weight estimation of polypeptide chains by electrophoresis in SDS-polyacrylamide gels. Biochem Biophys Res Commun. 1967 Sep 7;28(5):815–820. doi: 10.1016/0006-291x(67)90391-9. [DOI] [PubMed] [Google Scholar]
- Spiegelman S., Axel R., Schlom J. Virus-related RNA in human and mouse mammary tumors. J Natl Cancer Inst. 1972 Apr;48(4):1205–1211. [PubMed] [Google Scholar]
- Spiegelman S., Burny A., Das M. R., Keydar J., Schlom J., Travnicek M., Watson K. DNA-directed DNA polymerase activity in oncogenic RNA viruses. Nature. 1970 Sep 5;227(5262):1029–1031. doi: 10.1038/2271029a0. [DOI] [PubMed] [Google Scholar]
- Vaidya A. B., Black M. M., Dion A. S., Moore D. H. Homology between human breast tumour RNA and mouse mammary tumour virus genome. Nature. 1974 Jun 7;249(457):565–567. doi: 10.1038/249565a0. [DOI] [PubMed] [Google Scholar]
- Yagi M. J., Tomana M., Stutzman R. E., Robertson B. H., Compans R. W. Structural components of mouse mammary tumor virus. III. Composition and tryptic peptides of virion polypeptides. Virology. 1978 Dec;91(2):291–304. doi: 10.1016/0042-6822(78)90377-x. [DOI] [PubMed] [Google Scholar]
- Zacharius R. M., Zell T. E., Morrison J. H., Woodlock J. J. Glycoprotein staining following electrophoresis on acrylamide gels. Anal Biochem. 1969 Jul;30(1):148–152. doi: 10.1016/0003-2697(69)90383-2. [DOI] [PubMed] [Google Scholar]