Figure 3. Activity of P. aeruginosa neuraminidase mutations.
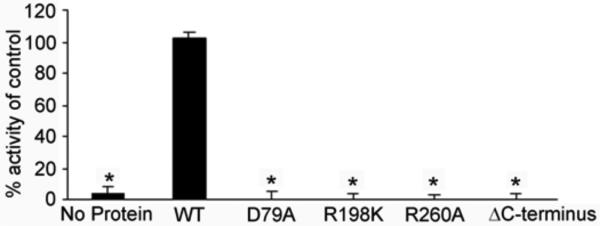
Site-directed mutations were made within the active site of P. aeruginosa neuraminidase or truncation in the C-terminus (deleting residues 334-438) and purified protein used to determine neuraminidase activity compared to wild-type enzyme (WT, control) using the fluorogenic substrate 2’-(4-methylumbelliferyl)-α-D-N-acetylneuraminic acid. *p-value <0.05.
