Abstract
Study Objectives:
The extant literature on predictors of treatment response to behavioral treatments for insomnia is equivocal and limited in scope. The current study examined demographic, clinical, and sleep characteristics as predictors of clinically significant treatment response to brief behavioral treatment of insomnia (BBTI) in older adults with insomnia.
Methods:
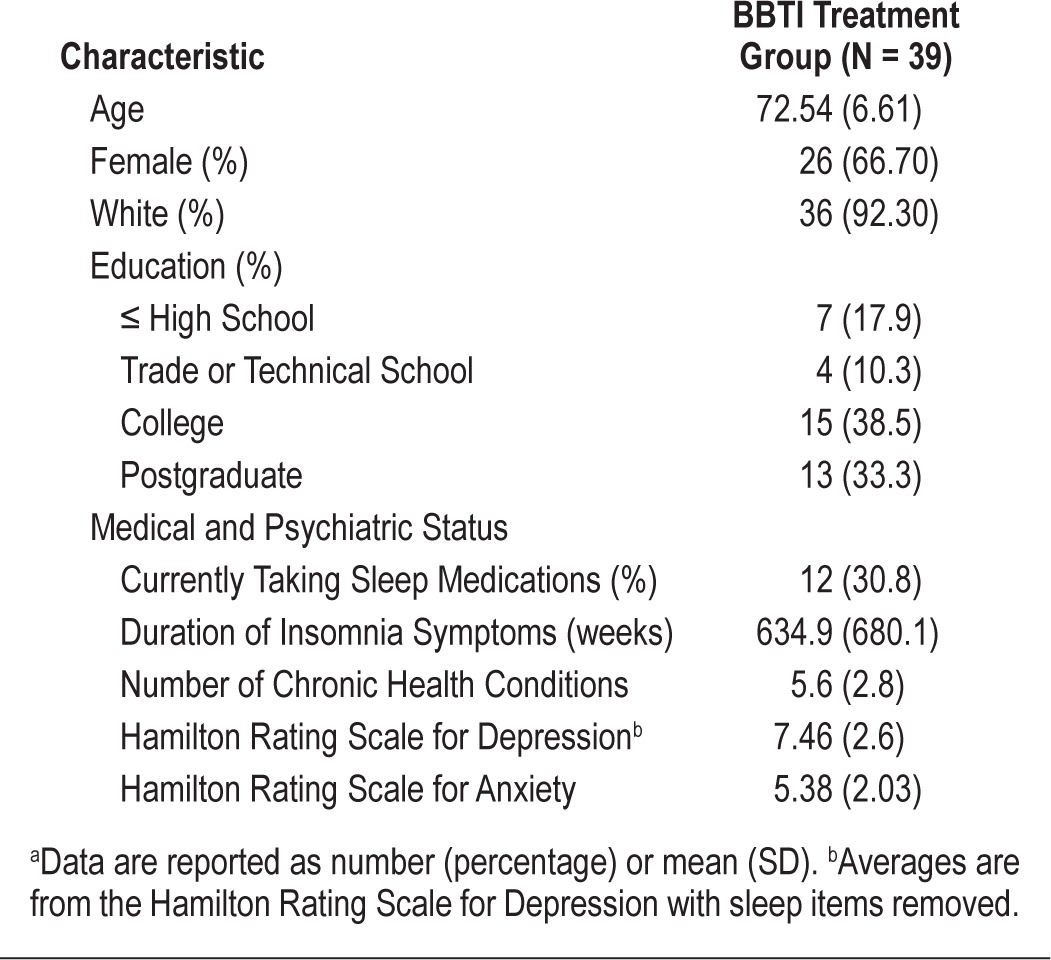
Thirty-nine older adults with insomnia (67% females, mean age: 72.54 years) were randomized to BBTI treatment. Treatment outcomes were defined according to 2 criteria: (1) “response,” defined as change in Pittsburgh Sleep Quality Index (PSQI) score ≥ 3 points or increase in sleep diary sleep efficiency ≥ 10%); or (2) remission, defined as absence of a clinical diagnosis of insomnia according to standard diagnostic criteria. Logistic regression examined whether baseline demographic, clinical, or sleep characteristics predicted treatment outcomes at 1 month follow-up.
Results:
Demographic variables did not predict treatment outcomes for either criterion. Higher anxiety, depression, poorer sleep quality, and longer polysomnography (PSG)-assessed sleep latency predicted greater likelihood of response at follow-up (p < 0.05). Longer sleep duration at baseline (measured by sleep diary and PSG) predicted greater likelihood of the remission at follow-up (p < 0.05).
Conclusion:
Patients with insomnia who have greater distress at baseline or prolonged sleep latency are more likely to show positive response to BBTI. In contrast, short sleepers at baseline are less likely to have resolution of insomnia diagnosis following BBTI, perhaps due to the sleep restriction component of the treatment. Identifying the characteristics that predict positive BBTI treatment outcomes can facilitate personalized behavioral treatments to improve outcomes.
Citation:
Troxel WM; Conrad TS; Germain A; Buysse DJ. Predictors of treatment response to brief behavioral treatment of insomnia (BBTI) in older adults. J Clin Sleep Med 2013;9(12):1281-1289.
Keywords: Insomnia, behavioral treatment, treatment response, predictors, cognitive-behavioral
Insomnia is a chronic and persistent sleep disorder which affects approximately 10% of the adult population, and up to 20% in geriatric populations.1,2 Over the past 20 years, robust and consistent evidence has demonstrated that behavioral interventions (under the umbrella of cognitive behavioral therapy for insomnia; CBTI) are comparably efficacious and perhaps more enduring than pharmacologic interventions.3 Despite the well-documented benefits of CBTI, pharmacotherapy remains the front-line treatment for insomnia in many primary care settings, in part due to the lack of trained CBTI clinicians. To address this challenge, our group recently demonstrated the efficacy of a variant of CBTI, called brief behavioral treatment of insomnia (BBTI),4 in a sample of older adults with insomnia. BBTI is shorter in duration than traditional CBTI and designed to be delivered by a nurse with limited training in sleep medicine. The improvements seen with BBTI (effect sizes ranging from 0.62-0.96 for quantitative sleep parameters) were comparable in magnitude to those reported in a meta-analysis of CBTI and other behavioral interventions for insomnia in older adults.5 As with traditional CBTI, however, there was considerable variability in treatment response to BBTI. In other words, though treatment was efficacious overall, not all patients demonstrated significant improvement. Identifying characteristics of individuals who are more or less likely to benefit from a given treatment is of critical clinical importance in order to maximize patient benefits and cost-effectiveness, and minimize side effects. Therefore, the purpose of the present study is to identify predictors of treatment response to BBTI.
BRIEF SUMMARY
Current Knowledge/Study Rationale: Research on predictors of treatment response following behavioral treatment of insomnia is equivocal and limited in scope. The current study includes a broad assessment of demographic, clinical, and sleep characteristics as predictors of treatment response to brief behavioral treatment of insomnia (BBTI) in a sample of older adults with insomnia.
Study Impact: Identifying patient characteristics that predict favorable treatment outcomes to behavioral treatment of insomnia is critical to tailor treatment efforts and optimize treatment outcomes. The current study demonstrated that higher levels of depression and anxiety predicted better treatment response to BBTI, whereas shorter sleep latency and shorter total sleep time predicted poorer treatment outcomes.
Although this is the first study to examine predictors of response to BBTI, previous research has examined the influence of several demographic, clinical, and psychiatric variables on treatment response to CBTI. For the most part, however, prior results are equivocal, perhaps due to methodological differences across studies. For instance, age has not been consistently related to treatment response, with a handful of studies reporting older age being related to poorer outcome6–8 and other studies showing no influence of age on treatment response,9–11 perhaps due to differences in study eligibility criteria (including a varying range of ages for “older” or “younger” participants) or statistical control for medical comorbidities, which are particularly common in older adults. Indeed, once screened for medical comorbidities, older patients have been shown to respond similarly to their younger counterparts.12 These findings suggest that older age does not portend poorer treatment response; however, comorbidities associated with increasing age may account for age differences in treatment outcome.
Differences in eligibility criteria may also account for equivocal findings with regard to baseline clinical characteristics. For instance, in a clinical effectiveness trial, which included patients representative of those presenting in clinical practice, Espie and colleagues found that higher levels of baseline depression and anxiety and initial insomnia severity predicted greater treatment response.13 In contrast, the majority of clinical trials of CBTI, which have been conducted in highly controlled research trials with more stringent eligibility criteria, have shown higher severity and longer duration of insomnia associated with poorer response,8 while still other studies have shown no relation to treatment response.7,14 Thus, given the conflicting nature of these results and that the vast majority of insomnia patients can be characterized as comorbid cases (particularly among older adults), further investigation of predictors of treatment response in samples more characteristic of the general insomnia population is warranted.
Aside from patient characteristics, the operational definition of treatment response varies widely and may influence the clinical significance of treatment response.15 To date, most studies assess treatment response via change in clinically relevant quantitative sleep parameters (e.g., sleep latency, wakefulness after sleep onset, sleep efficiency) measured via sleep diaries and/or actigraphy.7,16 Although these quantitative criteria have the utility of being well recognized and relatively well standardized in terms of research diagnostic criteria,17 their clinical utility is somewhat limited as current clinical diagnostic criteria are based on the clinical complaint of insomnia with daytime impairment, and do not specify quantitative parameters (e.g., sleep latency > 30 min). Moreover, quantitative criteria may not adequately reflect the patient's experience of clinically significant improvement. For instance, Currie and colleagues demonstrated that 57% of patients met treatment response criteria based on significant reductions in Pittsburgh Sleep Quality Index (PSQI) scores after 7 weeks of treatment; however, only 18% were considered fully recovered from their sleep problems, based on reliable change in PSQI scores and clinical criteria for normative values on diary-assessed quantitative sleep parameters (e.g., total sleep time, sleep efficiency) and PSQI scores < 5.18 Reimann and Perlis have also recently advocated for outcome data that can be interpreted in terms of clinical relevance, such as percentages of responders/nonresponders or those meeting/not-meeting insomnia diagnostic criteria at follow-up.19
Finally, few previous investigations have examined physiological predictors of treatment response. In a sample of middle-aged adults with insomnia, Krystal and Edinger found that lower peak delta and a more gradual decline of delta across NREM periods prior to treatment predicted a greater subjective response to CBTI.20 Given age-related changes in sleep architecture and micro-architecture, particularly with regard to the levels and slope of decline in delta activity and the putative role of homeostatic sleep pressure as a mechanism of change in behavioral insomnia treatments, examination of the role of delta activity as a predictor of treatment response in older adults may provide useful insights into how to further improve or refine behavioral insomnia treatments.
In summary, prior research on predictors of treatment response to CBTI have not identified a reliable set of predictors to treatment response, and no prior study has examined predictors of response to BBTI. Several methodological characteristics of the BBTI clinical trial offer unique opportunities to address unresolved issues in the extant literature on predictors of treatment response to CBTI. In particular, the BBTI trial includes older adults recruited from the community and ranging in age from 62 to 88 years, which offers the opportunity to examine predictors of treatment response in an older adult population. In addition, to maximize generalizability of the results and in contrast to most prior clinical trials of behavioral treatments of insomnia, the BBTI trial did not exclude based on the presence of other co-occurring medical or psychiatric conditions, which more accurately reflects the vast majority of insomnia cases. The current study incorporates a broader assessment of demographic, clinical, and physiological predictors of treatment response than has been considered in prior research. Finally, the current study includes treatment response criteria that are intended to more closely reflect clinically relevant domains of improvement.
METHODS
Overview
These data were collected as part of a study of older adults with chronic insomnia (symptoms present ≥ 1 month) and their response to a brief behavioral treatment for insomnia (BBTI; AG 20677; Buysse, PI). Detailed study procedures have been published previously.4,21 Briefly, participants were recruited from a single primary care practice in the Pittsburgh area or from community advertisements. Following screening and baseline assessments, participants were randomly assigned to an active treatment condition (BBTI) or information-control condition. Given the current study focus on predictors of BBTI treatment outcome, participants included in the present analyses (n = 39) were insomnia patients who were randomized to BBTI treatment. Predictors of treatment outcome were collected at the baseline assessment and included demographic and clinical characteristics assessed by questionnaires, and sleep characteristics assessed by sleep diaries, actigraphy, and polysomnography. Treatment response was determined after 4 weeks of treatment. The University of Pittsburgh Biomedical Institutional Review Board approved this study. All participants provided written informed consent.
Participants
Eligibility criteria required that participants be at least 60 years of age and meet the general criteria for insomnia in the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition, Text Revision (DSM-IV-TR)22 and the International Classification of Sleep Disorders, 2nd Edition (ICSD-2),23 A structured clinical interview was used to determine eligibility, i.e., the presence/absence of insomnia disorder and other sleep disorders. As part of the interview, we administered an insomnia symptom checklist (see supplemental material, Figure S3) to specifically examine DSM-IV and ICSD-2 criteria. Specifically, insomnia criteria included: presence of a sleep complaint lasting for at least one month (median duration of symptoms was 351 weeks; minimum = 35 weeks; maximum = 2,860 weeks); adequate opportunity and circumstances for sleep; and evidence of significant distress or daytime impairment. The checklist was administered at both time points and was used to determine the presence/absence of insomnia post-treatment.
To optimize the clinical relevance and generalizability of the study, participants were eligible if they had stable, co-occurring medical or psychiatric disorders. Therefore, most of our participants had comorbid insomnia. The following exclusion criteria were applied: presence of dementia (identified by history or a score < 25 on the Folstein Mini Mental Status Exam)24 or delirium; previously undiagnosed and untreated depressive, anxiety, psychotic, or substance use disorders (those with stably treated depressive and anxiety disorders were not excluded); untreated severe obstructive sleep apnea syndrome (apneahypopnea index [AHI] > 20), restless legs syndrome or other sleep disorders (those with stably-treated sleep disorders were not excluded); hospitalization within the past 2 weeks; ongoing chemotherapy or other cancer treatment; and terminal illness with life expectancy less than 6 months.
Intervention
Detailed descriptions of BBTI efficacy data and therapeutic guidelines have been previously reported.4,21 The manualized intervention consists of a single 45- to 60-min in-person, individual session, followed by a 30-min follow-up session 2 weeks later, and 20-min phone sessions after 1 and 3 weeks of treatment. All sessions were conducted by a master's level mental health nurse. BBTI shares many features of standard CBTI; however, it is distinct in its explicit behavioral focus (i.e., primary treatments are stimulus control and sleep restriction), its relatively short duration including 2 phone call sessions, its delivery by a nurse without prior training in behavioral sleep medicine, and the provision of a hard-copy workbook which includes the treatment rationale and specific written instructions for prescribed sleep behaviors. All intervention sessions were audiotaped. An independent evaluator randomly rated 33% of the audio-tapes using a checklist of the 4 treatment elements specified in the treatment manual to rate treatment fidelity. BBTI sessions contained 97% (SD = 3.2) of intended BBTI treatment elements.
Treatment Predictors
Baseline predictors of treatment outcome were assessed prior to treatment initiation and included data from interviews and questionnaires, sleep diaries, wrist actigraphy, and polysomnography (individual methods described below).
Demographics
Demographic characteristics including age, sex, marital status, race/ethnicity, and education were assessed by self-report.
Baseline Clinical Characteristics
Depressive symptoms were assessed with the 17-item Hamilton Rating Scale for Depression (HRSD).25 The HRSD is a clinician-administered interview scale that assesses the presence and severity of 17 symptoms of depression experienced in the past week using a varied response format ranging from 0-2 to 0-4 (with higher scores indicating greater depression severity), and exhibits well-documented reliability and validity.26 Anxiety symptoms were assessed using the Hamilton Anxiety Rating Scale (HRSA),27 a widely used and well-validated interview scale that assesses 14 symptoms of anxiety.28 Three items on the HRSD and 1 item on the HRSA pertaining to sleep disturbance were removed from all subsequent analyses to avoid confounding with the outcomes. Given research suggesting that people's beliefs and expectations with respect to behavioral treatments play a crucial role in shaping their experiences and outcomes of that treatment, we administered a modified, 4-item version of the Credibility and Expectancy Questionnaire (CEQ)29 to all participants prior to their first treatment session, but after a brief description of each treatment condition (BBTI or information-control was provided). Example items were: “How sensible/logical does this intervention seem?” and “how much improvement in your sleep do you think will occur because of this type of treatment?” The 4 items were standardized and summed, yielding a total score with excellent internal consistency (α = 0.85).
Medical comorbidities were evaluated with a comorbidity questionnaire developed at the Center for Research on Chronic Disorders at the University of Pittsburgh School of Nursing. This measure is adapted from the Charlson Comorbidity Index30 but includes a wider range of conditions, which were grouped into 17 categories (e.g., arthritis, cancer, coronary heart disease, diabetes).
Information regarding participants' use of medications known to affect sleep or wake functions (benzodiazepines, hypnotics, antidepressants, antipsychotics, anxiolytics, stimulants, antihistamines, decongestants, corticosteroids, diuretics) was collected via self-report and included as a potential predictor of treatment outcome.
Baseline Sleep Characteristics
The Pittsburgh Sleep Quality Index (PSQI)31 was used as a measure of global sleep quality. The PSQI is a widely-used, well-validated, self-report scale, used to assess sleep quality in the past month. Global PSQI score was utilized, with a total possible range from 0 (good sleep quality) to 21 (poor sleep quality). In the current sample, PSQI scores ranged from 6-16; (skewness = 0.34; kurtosis -0.61).
The Pittsburgh Sleep Diary (PghSD)32 is a prospective self-report measure of daytime activities, sleep behaviors, and sleep parameters. Previous research has demonstrated that the PghSD is sensitive to differences between sleep disorder patients and good sleeper controls, and to behavioral treatment effects in insomnia patients.32,33 The PghSD was administered via paper and pencil and collected for 2 weeks (mean = 13 days, SD = 1.49) at baseline. Baseline sleep diary sleep latency (SL), wakefulness after sleep onset (WASO), and total sleep time (TST), averaged over 2 weeks of baseline data collection, were evaluated as predictors of treatment outcome. Sleep diary sleep efficiency (SE; calculated as the ratio of time spent asleep/time in bed) collected after treatment was utilized in the definition of treatment response (as defined below).
Wrist actigraphy was measured with the Minimitter Acti-watch-64 device (Respironics, Inc., Murrysville, PA), which was worn concurrently with the collection of sleep diary data (mean = 13.86 days; SD = 1.49). Actigraphs are wrist-watch-sized, motion-sensitive monitors worn on the participant's nondominant arm that can be used to provide a behavioral measure of sleep-wake patterns. Actigraphy data were collected in 1-min epochs and analyzed with the validated Actiware Version 5.04 software program. Actigraphy variables evaluated as predictors of treatment outcome included SL, WASO, and TST (expressed in minutes). We used definitions provided by the Actiware software for these variables, which rely on values for bedtime and rise time from the sleep diary.
Visually scored and Quantitative EEG Sleep. Polysomnography (PSG) was conducted in participants' homes at their habitual sleep times using Compumedics Siesta units (Compumedics Limited, Abbotsford, Victoria, Australia). One screening PSG night was used to rule out severe obstructive sleep apnea or periodic limb movements (i.e., participants with apneahypopnea index [AHI] > 20 or periodic limb movement arousal index [PLMA-I] > 20, [according to American Academy of Sleep Medicine Task Force standards] were excluded). Visual sleep stage scoring was conducted in 20-sec epochs by trained PSG sleep technologists with established reliability, using standard scoring criteria34; this study was conducted prior to the AASM 2007 scoring rules. Visually scored PSG measures evaluated as predictors of treatment outcome were averaged over nights 2 and 3 and included SL, WASO, TST, AHI, and PLMA-I. In addition, quantitative EEG analysis35 was performed to quantify average power in the delta (0.05-4.0 Hz) range and slope of delta activity across the night, given previous associations between visually scored delta activity and treatment response.20 Modified periodograms were computed using the fast Fourier transform (FFT) of non-overlapping 4-sec epochs of the sleep EEG. NREM EEGs were binned into 5-min averages across each NREM period. Any 5 min epoch that had > 4 min of artifact was removed, as well as one peak epoch from each NREM period. The one peak epoch was removed since we often saw extreme blips at beginning or end of NREM period that we believed to be artifact due to sleep staging. The peak and average delta in each NREM period was calculated from the remaining epochs. A mixed effects repeated measures analysis of variance was use to model peak and average delta across NREM periods using random intercept and slope. A natural log transformation was used on both peak and average delta in the analyses. Models were run using whole group and each subject's model-based estimate of their intercept and slope as predictors in a logistic regression.
Treatment Outcomes
There are no universally accepted criteria for assessing response or remission in insomnia treatment studies.36 For the current study, outcomes were chosen because they met the following criteria which are thought to be indicative of clinically significant change: (1) outcomes correspond to approximately 1 standard deviation of the pretreatment values (i.e., a “large effect” according to Cohen's D of approximately 1.0); (2) outcomes are consistent with mean change values in published clinical trials; and (3) outcomes correspond to a change score of approximately -8 on the Insomnia Severity Index.37 Specifically, for the current study we focused on 2 binary treatment outcomes: (1) response/remission versus partial response or nonresponse; and (2) clinical remission, defined as the participant no longer meeting DSM-IV-TR and ICSD-2 criteria for insomnia disorder after treatment using a structured interview and checklist (described above). As reported in the BBTI efficacy study,4 the response/remission category consisted of those participants who had a change in PSQI score ≥ 3 points or increase in diary SE ≥ 10% (response) or remission defined as response criterion plus final PSQI score of < 5 and sleep diary SE of > 85%, corresponding to “good sleep” values.31 The partial response or nonresponse category consisted of those who showed improvement in PSQI or SE but worsening in the other measure or change in PSQI < 3 points and increase in sleep diary SE < 10%, respectively. For the current study, we refer to these categories as “response” (inclusive of response or remission) versus “non-response” (inclusive of partial or nonresponse).
Analyses
Sleep variables with non-normal distributions (i.e., sleep latency across all methods and diary-assessed WASO) were normalized using logarithmic transformations prior to analyses. Logistic regression models regressed each of the individual baseline variables on response or clinical remission criteria. Statistical analyses were conducted using IBM SPSS software, version 19. Statistical significance was set at a p-value of p < 0.05.
RESULTS
Table 1 describes sample characteristics for the 39 participants randomized to BBTI. As reported in Buysse et al., 67% of those randomized to BBTI met criteria for response/remission after 4 weeks of treatment, and 55% no longer met diagnostic criteria for insomnia after treatment.4
Table 1.
Baseline demographic and clinical characteristics of samplea
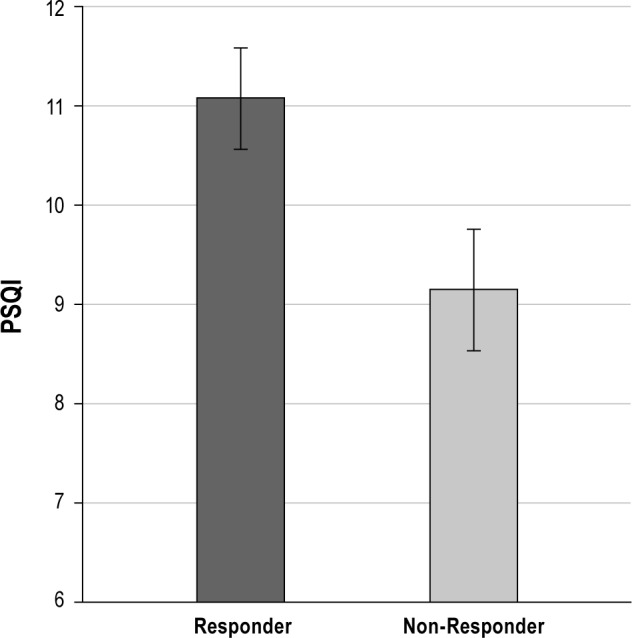
Logistic regression models which regressed baseline demographic, clinical, or sleep characteristics on response criteria are reported in Table 2. Higher levels of depression and anxiety were associated with higher likelihood of treatment response (as shown in Figures 1 and 2, respectively). In addition, poorer quality sleep at baseline, as indicated by higher PSQI scores, was associated with greater likelihood of treatment response. For visual purposes, Figure 3 displays mean baseline PSQI values for responders versus non-responders. There was also a significant association between PSG-assessed sleep latency and treatment response, such that patients with longer sleep latency at baseline were more likely to respond to BBTI. None of the demographic, sleep diary, actigraphy, or remaining PSG or clinical measures predicted treatment response.
Table 2.
Bivariate logistic regression predicting the odds of treatment response after brief behavioral treatment for insomnia (BBTI; N = 39)
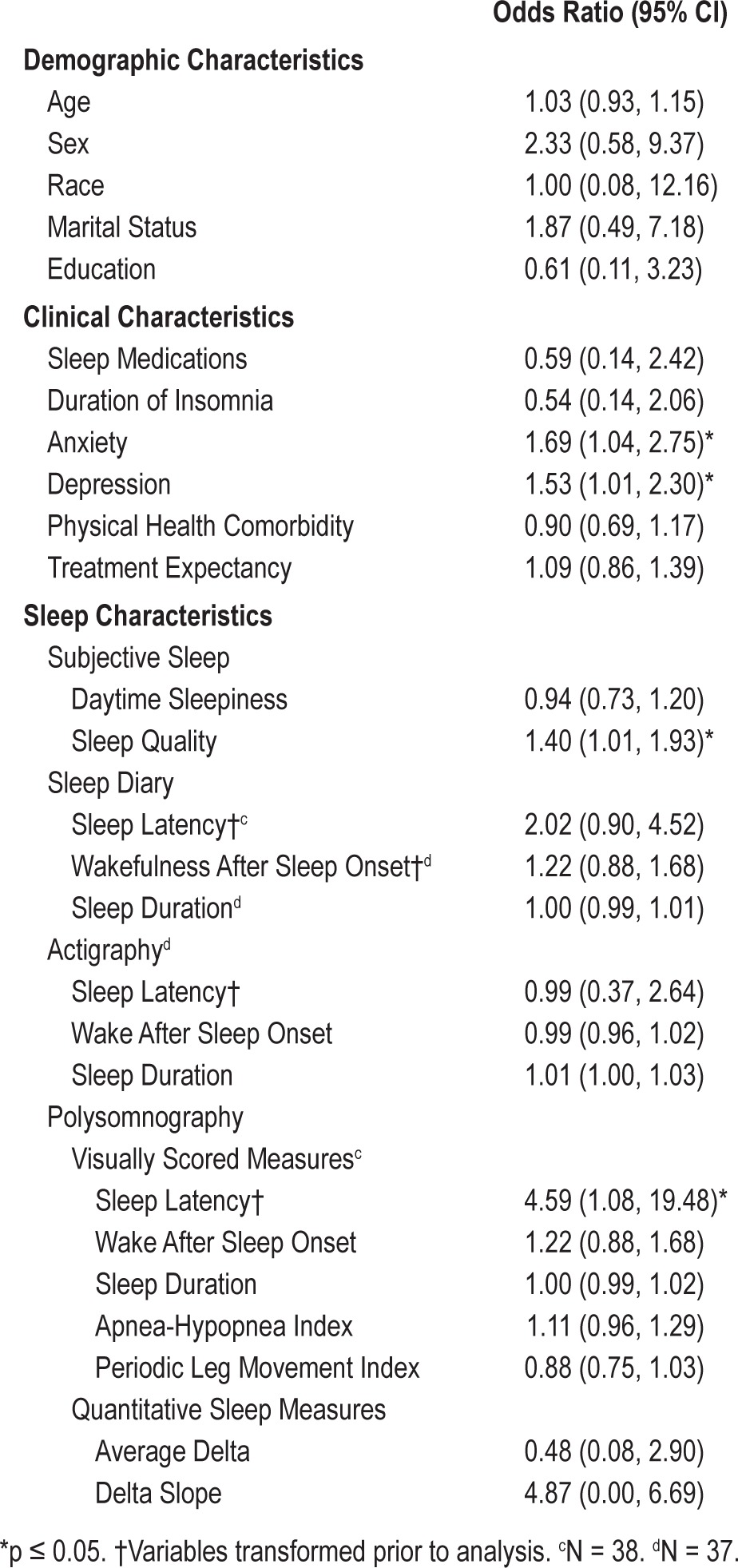
Figure 1. Mean Hamilton Rating Scale for Depression score according to responder status.
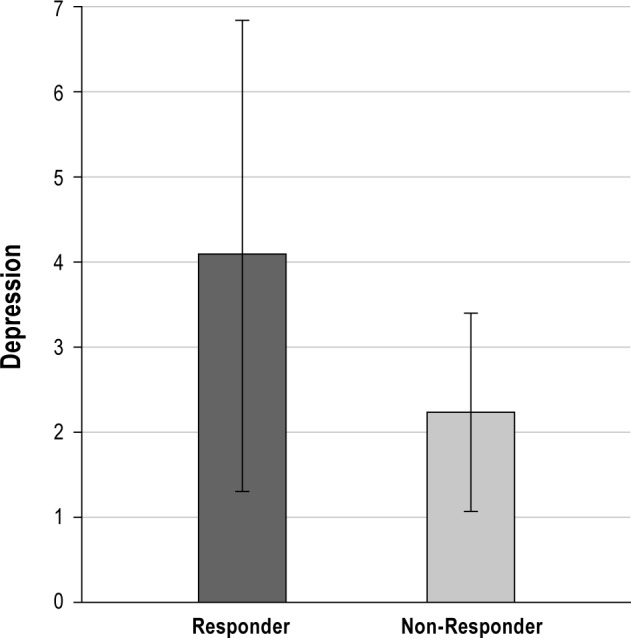
Figure 2. Mean Hamilton Rating Scale for Anxiety score according to responder status.
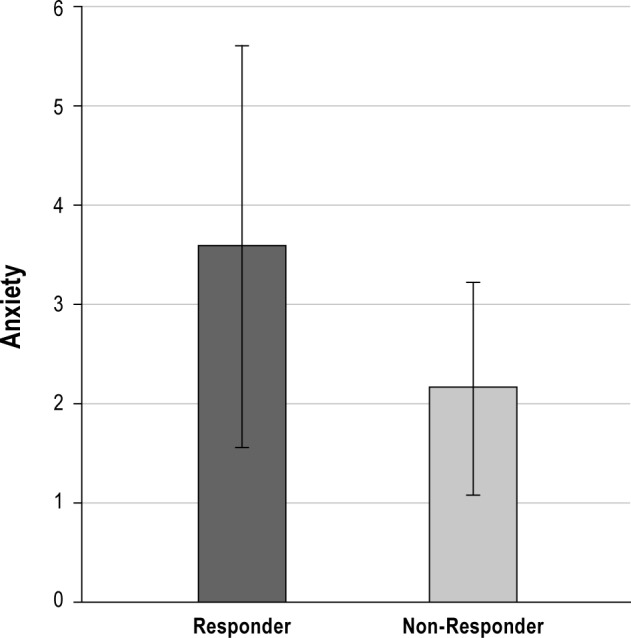
Figure 3. Mean PSQI score according to responder status.
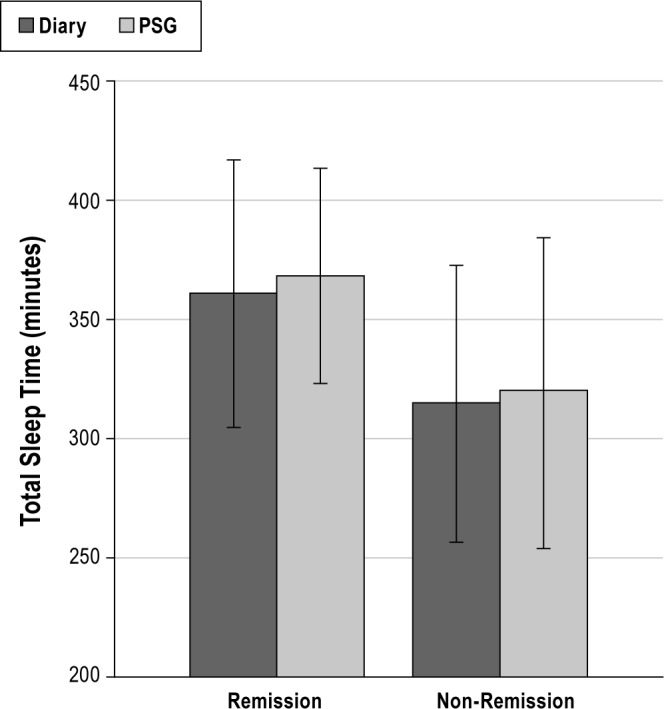
As shown in Table 3, the only significant predictors of clinical remission were total sleep time as assessed by sleep diary and in-home PSG. As shown in Figure 4, participants with longer PSG-assessed TST or longer diary-assessed TST at baseline were more likely to meet clinical remission criteria at post-treatment. Follow-up analyses which used the cutoff of ≤ 6 h of sleep (i.e., short sleepers) versus those with > 6 h of sleep, showed an increased odds of non-remission, among those defined as short sleepers by PSG (OR = 4.8; CI: 1.04-21.79) or diary (OR = 8.05; CI: 1.70-38.1). However, these analyses should be interpreted with caution due to the relatively small cell sizes for this categorical definition of short sleepers (n = 20 and n = 17, respectively, for PSG and diary), and the wide confidence interval.
Table 3.
Bivariate logistic regression predicting the odds of remission after brief behavioral treatment for insomnia (BBTI; N = 39)
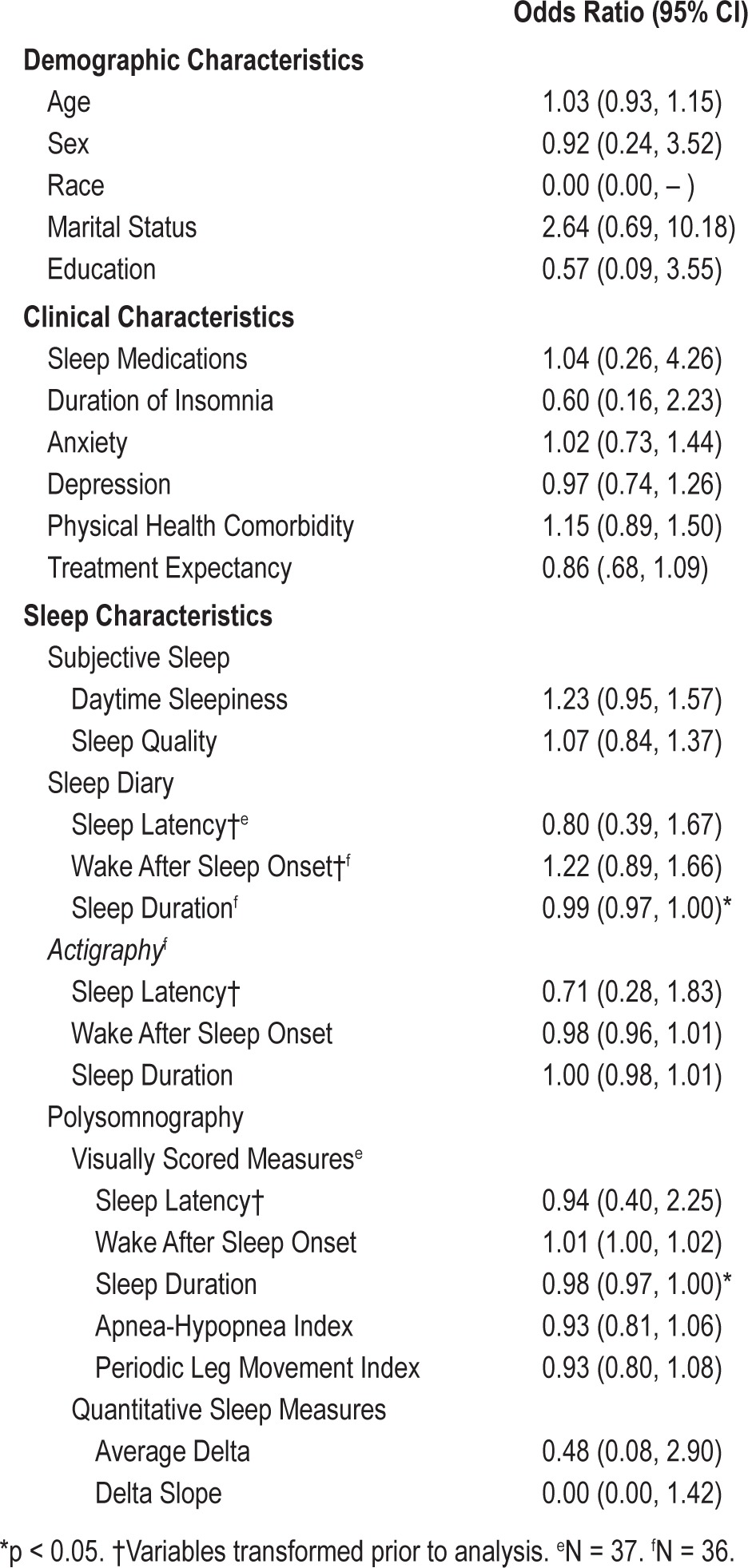
Figure 4. Diary or PSG-assessed total sleep time according to remission status.
DISCUSSION
Despite the well-documented efficacy of CBT for insomnia, behavioral treatments remain under-utilized, in part due to the lack of specialty trained clinicians. Such challenges to dissemination have motivated considerable efforts to develop variants of CBTI that can be more easily disseminated to broader clinical practice.38 BBTI has been shown to be efficacious for the treatment of older adults, most of whom have other comorbid medical or psychiatric conditions, with effect sizes comparable to those of CBTI. Although BBTI has been shown to be efficacious overall, this is the first study to examine a wide range of potential predictors of treatment response to BBTI.
Consistent with prior research on predictors of response to CBTI, we found no reliable evidence for differential treatment response according to demographic characteristics. However, these results may be due to the fact that the sample was restricted in terms of age and composed primarily of educated, Caucasian older adults. Although a handful of previous studies6–8,14 have indicated poorer treatment response among older adults versus younger adults, these findings may be more indicative of higher rates of co-occurring medical or psychiatric conditions in older adults. A strength of the current study was that to maximize generalizability of the findings to older adults with insomnia, we did not exclude participants with stable or treated co-occurring medical or psychiatric conditions or mild to moderate OSA or PLMs. The presence of such comorbidities also did not predict treatment response in this sample. In contrast, consistent with Espie effectiveness trial,13 higher clinical distress at pre-treatment, as indicated by higher depression and anxiety scores and poorer sleep quality, were associated with greater likelihood of meeting the response criteria at follow-up. Importantly, these distress characteristics were only associated with the more subjectively defined response criteria, but not by the insomnia criteria defined by structured interview, whereas PSG- or diary-assessed short sleep duration predicted the clinician-assessed outcome of remission. The fact that distress measures predicted better treatment response may reflect regression to the mean (i.e., greater opportunity for improvement with higher baseline values) or perhaps greater motivation among this subset who is most distressed by their insomnia. However, supplemental analyses conducted in the control condition demonstrated that among patients in the information-control condition, higher PSQI scores at baseline were associated with poorer treatment response (see Figure S2), which argues against regression to the mean. We also found that PSG-assessed prolonged sleep latency at baseline predicted higher likelihood of treatment response in the BBTI group, perhaps again due to greater motivation to change as well as opportunity for improvement, given that behavioral techniques including sleep compression are particularly effective at reducing sleep latency.
These findings are in contrast, to findings for the remission criteria, based on clinician-assessed diagnostic criteria, which showed that longer sleep diary and PSG-assessed total sleep time predicted greater likelihood of remission. Actigraphy-assessed sleep duration showed a similar pattern of results for remission, but did not reach statistical significance. Actigraphy or diary-assessed sleep latency and WASO were not associated with response or remission. These findings regarding short sleep duration have important clinical implications because they suggest that the possibility that individuals with shorter sleep durations may benefit less from behavioral sleep treatments (including BBTI), which utilize sleep restriction as a primary component of treatment. For safety reasons, including increased risk of falls associated with short sleep duration in older adults,39 the BBTI protocol did not restrict time in bed less than 6 hours per night, even if pretreatment total sleep time is estimated at 6 hours. Thus, given these safety constraints, the strength of the sleep restriction component may be diminished in these patients. Recent evidence from Vgontzas' laboratory suggests that there may be a synergistic effect of insomnia with short sleep duration on a wide variety of adverse outcomes, including poorer cognitive functioning and mortality.40,41 Thus, insomnia patients presenting with short sleep durations present a specific clinical challenge, given that they may fail to benefit as much from behavioral treatments of insomnia and are at greater risk for associated morbidities. On the other hand, the short duration of follow-up in this study (4 weeks) may have contributed to the finding linking short sleep duration with poor treatment outcome. For these patients, longer follow-up periods may be necessary to allow sufficient time for the benefits of sleep restriction to be realized. In contrast to the findings of Krystal and Edinger,20 we did not find evidence for an effect of overall delta activity or slope of delta activity across the night on treatment outcomes. Several methodological differences may account for the discrepancy in results. In particular, Krystal's findings were based on a smaller sample (N = 16) of primarily middle-aged adults (mean age = 56), whereas the current findings were based on a sample of 39 older adults all over the age of 60 (mean age = 72). Given age-related declines in peak delta activity and blunted delta dynamics (i.e., lesser slope) throughout the night, our findings which were restricted to older adults may reflect a lack of range in delta activity and subsequent reduction in power. In addition, the two studies used similar, but not identical, methods for calculating delta EEG activity. Nevertheless, given the putative role of homeostatic sleep pressure as a mechanism of change in behavioral sleep treatments, future research is needed to examine the impact of sleep micro-architecture and dynamics in broader clinical samples.
These findings must be interpreted within the context of study limitations. First, findings may not generalize beyond older, predominantly Caucasian adults with insomnia, who were recruited from the community or a primary care practice, and who volunteered to participate in research. Second, although the relatively inclusive recruitment strategy is a strength of the study, the enhanced generalizability of results also introduces greater heterogeneity and potential confounds, such as the inclusion of patients with mild to moderate levels of sleep disordered breathing or periodic leg movements, which may have influenced the results. Although, the study characterized a wide range of pretreatment demographic, clinical, sleep, and neurophysiologic indicators of treatment response, we did not measure all potentially informative treatment predictors, such as motivation for change (although we did assess treatment expectancies, which was not related to outcome). As previously mentioned, the relatively short follow-up (4 weeks) may have implications for the results, particularly with regard to the sleep duration finding. There are limitations with regard to the definition criteria for treatment response. Specifically, treatment response was based, in part, on change in PSQI scores, which is a general measure of sleep quality, rather than a measure specific to insomnia severity, such as the Insomnia Severity Index (ISI).37 However, only a subset of the sample (N = 17) completed the ISI. Finally, the magnitude of observed effects may be partially attributable to the use of a single clinician. Whether similar effects would be observed across different clinicians, with different professional backgrounds and varying levels of experiences cannot be ascertained. On the other hand, delivery by a single therapist has the advantage of minimizing inter-therapist variability.
These limitations notwithstanding, the current findings contribute to our understanding of in whom behavioral treatments for insomnia are most likely to benefit. This question is absolutely critical as there continues to be a substantial gap between the solid evidence base supporting behavioral treatments for insomnia and the actual use of such treatments in clinical practice. Our findings also suggest that even complex insomnia cases, including those with comorbid mood or sleep disorders or medical conditions may benefit from a brief behavioral treatment delivered by a mental health nurse without prior training in sleep medicine, rather than a doctoral-level clinical psychologist. These findings also provide convergent evidence from sleep diaries and PSG to suggest that insomnia patients with short sleep duration or with short sleep latency may be a specific subset of the patient population that requires different treatment approaches, with more extended follow-up, such as adjunctive pharmacotherapy or multi-component behavioral strategies which rely more on cognitive techniques (e.g., thought restructuring) or other behavioral techniques (e.g., relaxation or mindfulness-based approaches). Alternatively, it is possible that these patients would benefit from the sleep restriction component of BBTI if limits regarding the minimum time in bed (set at 6 hours for this protocol) were removed; however, safety issues are a concern. In summary, identifying predictors of treatment response to behavioral treatments for insomnia is critical in order to refine treatment algorithms to optimize treatment response, improve patient adherence and satisfaction, most efficiently allocate resources (including specialty trained clinicians), and ultimately reduce the economic and public health burden of insomnia.
DISCLOSURE STATEMENT
This was not an industry supported study. Funding for this research was provided by National Institutes of Health grants AG020677 (PI: Timothy Monk, Ph.D., D.Sc.), MH024652 (PI: Daniel Buysse, M.D.), RR024153 (PI: Daniel Buysse, M.D.), and K23HL093220 (PI: Wendy M. Troxel, Ph.D.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Dr. Buysse serves as a paid consultant for Merck, Philips, and Transcept Pharmaceuticals, Inc., and he has been paid for lectures at international, non-CME educational meetings supported by Servier and Astellas. The other authors have indicated no financial conflicts of interest.
ABBREVIATIONS
- AASM
American Academy of Sleep Medicine
- AHI
apnea-hypopnea index
- BBTI
brief behavioral treatment of insomnia
- CBTI
cognitive behavioral therapy for insomnia
- CCI
Charlson Comorbidity Index
- CEQ
Credibility and Expectancy Questionnaire
- DSM-IV-TR
Diagnostic and Statistical Manual of Mental Disorders 4th Edition Text Revision
- EEG
electroencephalography
- FFT
fast Fourier transformation
- HRSA
Hamilton Rating Scale for Anxiety
- HRSD
Hamilton Rating Scale for Depression
- ICSD-2
International Classification of Sleep Disorders 2nd Edition
- ISI
Insomnia Severity Index
- NREM
non-rapid eye movement
- PghSD
Pittsburgh Sleep Diary
- PLMA-I
periodic leg movement with arousal index
- PSG
polysomnography
- PSQI
Pittsburgh Sleep Quality Index
- SE
sleep efficiency
- SL
sleep latency
- TST
total sleep time
- WASO
wakefulness after sleep onset
SUPPLEMENTAL MATERIAL
Predictors of Outcomes for Participants Randomized to Information-Control (IC) Condition
Primary analyses focused on predictors of treatment response to the active treatment group (i.e., BBTI); however, we conducted supplemental analyses in the group randomized to IC in order to determine whether the same factors that predicted treatment response or remission in the active treatment condition (i.e., depression, anxiety, PSQI, PSG assessed sleep latency, or diary or PSG-assessed sleep duration) also contributed to improvements in the IC condition. There were no significant predictors of the response definition in the IC condition. The only significant predictor of remission in the IC group was PSQI (OR = 2.60; CI: 1.20, 5.63). However, in contrast to the BBTI condition, in the IC condition higher levels of PSQI scores at baseline were associated with lesser likelihood of meeting remission criteria post-treatment (see Figure S2).
Subjective-Objective Discrepancies in Sleep as Predictors of Response/Remission after BBTI
We examined the absolute difference in PSG and diary-reported SL, WASO, and TST as predictors of response or remission in the BBTI group. As shown in Table S1 and Table S2, none of the discrepancy scores predicted either outcome.
Objective-Subjective Discrepancies in Sleep as Predictors of Treatment Response after BBTI
Objective-Subjective Discrepancies in Sleep as Predictors of Remission after BBTI
Napping as a Predictor of Treatment Response or Remission after BBTI
Participants reported whether or not they took nap(s) in their daily diaries. We calculated the average number of naps over the dairy recording period by dividing the number of naps by the number of days of diary collection; values ranged from 0 to 1.15, with 24% of the sample reporting no naps over the diary recording period. Average number of naps was not associated with treatment response [OR = 0.25; CI; 0.03, 2.21] or remission [OR = 2.08; CI; 0.26, 16.9]. In addition, we examined whether napping accounted for the relationship between short sleep duration and treatment remission, by adding average number of naps to the logistic regression models with diary or PSG-sleep duration as the predictor of remission. Controlling for napping did not attenuate either of these significant results reported in Table 2 of the manuscript).
REFERENCES
- 1.Foley DJ, Monjan AA, Brown SL, Simonsick EM, Wallace RB, Blazer DG. Sleep complaints among elderly persons: an epidemiologic study of three communities. Sleep. 1995;18:425–32. doi: 10.1093/sleep/18.6.425. [DOI] [PubMed] [Google Scholar]
- 2.Ford DE, Kamerow DB. Epidemiologic study of sleep disturbances and psychiatric disorders. An opportunity for prevention? JAMA. 1989;262:1479–84. doi: 10.1001/jama.262.11.1479. [DOI] [PubMed] [Google Scholar]
- 3.Morin CM, Bootzin RR, Buysse DJ, Edinger JD, Espie CA, Lichstein KL. Psychological and behavioral treatment of insomnia: An update of recent evidence (1998-2004) Sleep. 2006;29:1398–414. doi: 10.1093/sleep/29.11.1398. [DOI] [PubMed] [Google Scholar]
- 4.Buysse DJ, Germain A, Moul DE, et al. Efficacy of brief behavioral treatment for chronic insomnia in older adults. Arch Intern Med. 2011;171:887–95. doi: 10.1001/archinternmed.2010.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Irwin MR, Cole JC, Nicassio PM. Comparative meta-analysis of behavioral interventions for insomnia and their efficacy in middle-aged adults and in older adults 55+ years of age. Health Psychol. 2006;25:3–14. doi: 10.1037/0278-6133.25.1.3. [DOI] [PubMed] [Google Scholar]
- 6.Alperson J, Biglan A. Self administered treatment of sleep onset insomnia and the importance of age. Behav Ther. 1979;10:337–46. [Google Scholar]
- 7.Edinger JD, Stout AL, Hoelscher TJ. Cluster analysis of insomniacs' MMPI profiles: relation of subtypes to sleep history and treatment outcome. Psychosom Med. 1988;50:77–87. doi: 10.1097/00006842-198801000-00010. [DOI] [PubMed] [Google Scholar]
- 8.Gagne A, Morin CM. Predicting treatment response in older adults with insomnia. J Clin Geropsychol. 2001;7:131–43. [Google Scholar]
- 9.Chambers MJ, Alexander SD. Assessment and prediction of outcome for a brief behavioral insomnia treatment program. J Behav Ther Exp Psychiatry. 1992;23:289–97. doi: 10.1016/0005-7916(92)90051-j. [DOI] [PubMed] [Google Scholar]
- 10.Espie CA, Inglis SJ, Tessier S, Harvey L. The clinical effectiveness of cognitive behaviour therapy for chronic insomnia: Implementation and evaluation of a sleep clinic in general medical practice. Behav Res Ther. 2001;39:60. doi: 10.1016/s0005-7967(99)00157-6. [DOI] [PubMed] [Google Scholar]
- 11.Van Houdenhove L, Buyse B, Gabriels L, Van den Bergh O. Treating primary insomnia: Clinical effectiveness and predictors of outcomes on sleep, daytime function and health-related quality of life. J Clin Psychol Med Settings. 2011;18:312–21. doi: 10.1007/s10880-011-9250-7. [DOI] [PubMed] [Google Scholar]
- 12.Morin CM, Kowatch RA, Barry T, Walton E. Cognitive-behavior therapy for late-life insomnia. J Consult Clin Psychol. 1993;61:137–46. doi: 10.1037//0022-006x.61.1.137. [DOI] [PubMed] [Google Scholar]
- 13.Espie CA, Inglis SJ, Harvey L. Predicting clinically significant response to cognitive behavior therapy for chronic insomnia in general motor practice: Analyses of outcome data at 12 months posttreatment. J Consult Clin Psychol. 2001;69:58–66. doi: 10.1037//0022-006x.69.1.58. [DOI] [PubMed] [Google Scholar]
- 14.Lacks P, Powlishta K. Improvement following behavioral treatment for insomnia: Clinical significance, long-term maintenance, and predictors of outcome. Behav Ther. 1989;20:117–34. [Google Scholar]
- 15.Vincent N, Penner S, Lewycky S. What predicts patients' perceptions of improvement in insomnia? J Sleep Res. 2006;15:301–8. doi: 10.1111/j.1365-2869.2006.00529.x. [DOI] [PubMed] [Google Scholar]
- 16.Bliwise DL, Friedman L, Nekich JC, Yesavage JA. Prediction of outcome in behaviorally based insomnia treatments. J Behav Ther Exp Psychiatry. 1995;26:17–23. doi: 10.1016/0005-7916(94)00073-u. [DOI] [PubMed] [Google Scholar]
- 17.Lichstein KL, Durrence HH, Taylor DJ, Bush AJ, Riedel BW. Quantitative criteria for insomnia. Behav Res Ther. 2003;41:427–45. doi: 10.1016/s0005-7967(02)00023-2. [DOI] [PubMed] [Google Scholar]
- 18.Currie SR, Wilson KG, Curran D. Clinical significance and predictors of treatment response to cognitive-behavior therapy for insomnia secondary to chronic pain. J Behav Med. 2002;25:135–53. doi: 10.1023/a:1014832720903. [DOI] [PubMed] [Google Scholar]
- 19.Riemann D, Perlis ML. The treatments of chronic insomnia: a review of benzodiazepine receptor agonists and psychological and behavioral therapies. Sleep Med Rev. 2009;13:205–14. doi: 10.1016/j.smrv.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 20.Krystal AD, Edinger JD. Sleep EEG predictors and correlates of the response to cognitive behavioral therapy for insomnia. Sleep. 2010;33:669–77. doi: 10.1093/sleep/33.5.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Troxel WM, Germain A, Buysse DJ. Clinical management of insomnia with Brief Behavioral Treatment (BBTI) Behav Sleep Med. 2012;10:266–79. doi: 10.1080/15402002.2011.607200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.American Psychiatric Association. Diagnostic and statistical manual of mental disorders (DSM-IV-TR). fourth edition, text revision. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 23.American Academy of Sleep Medicine. International classification of sleep disorders, second edition (ICSD-2): diagnostic and coding manual. Westchester, IL: American Academy of Sleep Medicine; 2005. [Google Scholar]
- 24.Folstein MF, Folstein SW, McHugh PR. Mini-mental state: A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 25.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prien RF, Carpenter LL, Kupfer DJ. The definition and operational criteria for treatment outcome of major depressive disorder: A review of the current research literature. Arch Gen Psychiatry. 1991;48:796–800. doi: 10.1001/archpsyc.1991.01810330020003. [DOI] [PubMed] [Google Scholar]
- 27.Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32:50–5. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 28.Shear MK, Vander Bilt J, Rucci P, et al. Reliability and validity of a structured interview guide for the Hamilton Anxiety Rating Scale. Depress Anxiety. 2001;13:166–78. [PubMed] [Google Scholar]
- 29.Borkovec TD, Nau SD. Credibility of analogue therapy rationales. J Behav Ther Exp Psychiatry. 1972;3:257–60. [Google Scholar]
- 30.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies. J Chron Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 31.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 32.Monk TH, Reynolds CF, Kupfer DJ, et al. The Pittsburgh Sleep Diary. J Sleep Res. 1994;3:111–20. [PubMed] [Google Scholar]
- 33.Germain A, Moul DE, Franzen PL, et al. Effects of a brief behavioral treatment for late-life insomnia: Preliminary findings. J Clin Sleep Med. 2006;2:403–6. [PubMed] [Google Scholar]
- 34.Rechtschaffen A, Kales A. Washington DC: US Government Printing Office; 1968. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. [Google Scholar]
- 35.Brunner DP, Vasko RC, Detka CS, Monahan JP, Reynolds CF, Kupfer DJ. Muscle artifacts in the sleep EEG: Automated detection and effect on all-night EEG power spectra. J Sleep Res. 1996;5:155–64. doi: 10.1046/j.1365-2869.1996.00009.x. [DOI] [PubMed] [Google Scholar]
- 36.Buysse DJ, Ancoli-Israel S, Edinger JD, Lichstein KL, Morin CM. Recommendations for a standard research assessment of insomnia. Sleep. 2006;29:1155–73. doi: 10.1093/sleep/29.9.1155. [DOI] [PubMed] [Google Scholar]
- 37.Yang M, Morin CM, Schaefer K, Wallenstein GV. Interpreting score differences in the Insomnia Severity Index: using health-related outcomes to define the minimally important difference. Curr Med Res Opin. 2009;25:2487–94. doi: 10.1185/03007990903167415. [DOI] [PubMed] [Google Scholar]
- 38.Perlis ML, Smith MT. How can we make CBTI and other BSM services widely available? J Clin Sleep Med. 2008;4:11–3. [PMC free article] [PubMed] [Google Scholar]
- 39.Stone KL, Ancoli-Israel S, Blackwell T, et al. Actigraphy-measured sleep characteristics and risk of falls in older women. Arch Intern Med. 2008;168:1768–75. doi: 10.1001/archinte.168.16.1768. [DOI] [PubMed] [Google Scholar]
- 40.Fernandez-Mendoza J, Calhoun S, Bixler EO, et al. Insomnia with objective short sleep duration is associated with deficits in neuropsychological performance: a general population study. Sleep. 2010;33:459–65. doi: 10.1093/sleep/33.4.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vgontzas AN, Liao D, Pejovic S, et al. Insomnia with short sleep duration and mortality: the Penn State cohort. Sleep. 2010;33:1159–64. doi: 10.1093/sleep/33.9.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Objective-Subjective Discrepancies in Sleep as Predictors of Treatment Response after BBTI
Objective-Subjective Discrepancies in Sleep as Predictors of Remission after BBTI


