Abstract
Objective:
The population-based University of Washington Twin Registry (UWTR) was used to examine (1) genetic influences on chronobiology and (2) whether these genetic factors influence alcohol-use phenotypes.
Methods:
We used a reduced Horne-Östberg Morningness-Eveningness Questionnaire (rMEQ) to survey UWTR participants for diurnal preference. Frequency and quantity of alcohol use, as well as binge drinking (6+ drinks per occasion), were assessed on a 5-point Likert scale. Both diurnal preference and alcohol use were self-reported. Twin data were analyzed by using structural equation models.
Results:
The sample consisted of 2,945 participants (mean age = 36.4 years), including 1,127 same-sex and opposite-sex twin pairs and 691 individual twins. The rMEQ range was 4-25, with a mean score of 15.3 (SD 4.0). Diurnal “morning types” comprised 30.7% (N = 903) of participants, while 17.4% (N = 513) were “evening types.” Regarding alcohol use, 21.2% (N = 624) reported never drinking. Among drinkers, 35.7% (N = 829) reported ≥ 3 drinks per occasion and 48.1% (N = 1,116) reported at least one instance of binge drinking. Genetic influences accounted for 37% of the variance in diurnal preference, with the remaining 63% due to non-shared environmental influences. Genetic propensities toward diurnal eveningness were significantly associated with increased alcohol quantity (β = -0.17; SE = 0.05, p < 0.001) and increased binge drinking (β = -0.19; SE = 0.04, p < 0.001), but not with frequency of alcohol use. Environmental paths between diurnal preference and alcohol use phenotypes were not significant.
Conclusions:
Genetic influences on diurnal preference confer elevated risk for problematic alcohol use, including increased quantity and binge drinking. Differences in circadian rhythm may be an important and understudied pathway of risk for genetic influences on alcohol use.
Citation:
Watson NF; Buchwald D; Harden KP. A twin study of genetic influences on diurnal preference and risk for alcohol use outcomes. J Clin Sleep Med 2013;9(12):1333-1339.
Keywords: Twins, monozygotic, dizygotic, alcohol, circadian, diurnal
Circadian rhythms are biological processes, such as the human sleep-wake cycle, with an endogenous, entrainable oscillation of roughly 24 hours. The hypothalamic suprachiasmatic nucleus is the primary circadian pacemaker, ensuring a proper duration and consistent timing of sleep. Healthy sleep arises from an effective interaction between the sleep homeostat, which increases sleep propensity as a function of prior wakefulness, and the 24-hour circadian alerting signal generated by the suprachiasmatic nucleus.1 In practical terms, this interaction results in a wide range of circadian functioning (also known as “diurnal preference” or “chrono-type”), from morning types to evening types. Morning types go to bed early and function best in the early daytime hours, whereas evening types go to bed in the early morning hours and function best at later times in the day or evening.2 Twin and molecular genetic studies consistently show that diurnal preference is influenced by genetic factors, with heritability between 40% and 54%.3–12
Recent research has focused on the role of circadian rhythms in health and disease. Circadian clock disruptions, often observed in shift work disorder, are associated with numerous medical conditions, including cardiovascular disease,13–15 cancer,16–18 and untoward pregnancy outcomes.19,20 It is less clear whether diurnal preference alone portends specific health outcomes. Preliminary evidence suggests that evening-type diurnal preference is associated with poor diet21 and depression22 and has adverse effects on measures of quality of life in adolescents.23 We previously showed an association between evening type and habitual short and long sleep duration in a twin sample.12 Since both short and long sleep are associated with adverse health outcomes,24–34 these findings suggest that the evening type may represent an endophenotype for poor health.
BRIEF SUMMARY
Current Knowledge/Study Rationale: Preliminary evidence suggests that diurnal preference is a contributing psychological factor in a multi-causal model of alcohol consumption. The extent to which the relationship between diurnal preference and alcohol use is driven by common underlying genetic variants is unknown.
Study Impact: This study shows that genetic factors favoring evening-type diurnal preference confer increased risk of binge drinking and increased alcohol consumption. This finding suggests that biological differences in circadian rhythm may be an important pathway of risk for genetic factors that promote alcohol use.
Preliminary evidence also suggests that diurnal preference influences alcohol use, such that evening types consume more alcohol than morning types.35–37 Alcohol consumption is associated with a single-nucleotide polymorphism in NPAS2, a gene involved in the autoregulatory transcription/translation feedback loop that drives circadian rhythmicity.38 Evening type is also correlated with novelty-seeking, which is thought to be associated with behavior activation by low basal dopaminergic activity in the brain.39,40 This may lead to addictive behaviors, such as alcohol abuse or dependence, in an effort to enhance dopamine levels.41 These preliminary studies suggest that diurnal preference is a contributing psychological factor in a multi-causal model of alcohol consumption. The extent to which the relationship between diurnal preference and alcohol use is driven by common underlying genetic variants has yet to be determined. Therefore, the goals of this twin study were to (1) determine the magnitude of genetic and environmental influences on diurnal preference and (2) evaluate the extent to which genetic influences on diurnal preference confer risk for alcohol use. We hypothesized that genetic predispositions toward eveningness would be associated with more problematic alcohol use, although the dearth of previous behavioral genetic research on this topic made our hypotheses necessarily speculative.
METHODS
University of Washington Twin Registry
The University of Washington Twin Registry is a community-based sample of twins constructed with data provided by the Washington State Department of Licensing. All data collection procedures were approved by the University of Washington Institutional Review Board. The minimum age for participation is 18, and < 5% of participants are older than age 66. As of April 2013, the Registry contained more than 7,500 twin pairs. Participants' zygosity is determined by using validated self-report methods, with an accuracy ≥ 95%.42,43 Every participant completes a recruitment survey. In 2006 and 2008, an additional health survey that included items on diurnal preference and alcohol use was mailed to more than 4,000 enrolled twins. Further details on the characteristics of Registry participants are available elsewhere.44,45
Our study sample consisted of 2,945 individuals, including 1,127 twin pairs (200 monozygotic [MZ] male [17.7%], 82 dizygotic [DZ] male [7.3%], 432 MZ female [38.3%], 215 DZ female [19.1%], and 198 DZ opposite-sex [17.6%]), as well as 691 individual twins who participated without their co-twins. All twin pairs were raised together. Data from incomplete twin pairs were retained because they inform the within-person correlations between diurnal preference and alcohol use. Data collection procedures were approved by the University of Washington Institutional Review Board. The sex of individual twins closely mirrored that observed in complete twin pairs.
Measures
Diurnal Preference
Diurnal preference was measured by using the reduced Morningness-Eveningness Questionnaire (rMEQ),46 a shortened version of the Horne and Östberg Morningness-Eveningness Questionnaire.47 The rMEQ contains 5 items that assess aspects of the morning-eveningness dimension (for example, “at what time in the evening do you feel tired and in need of sleep?”), rated on a 5-point Likert scale. Responses to each question are summed to give a total rMEQ score between 4 and 25, with higher scores indicating stronger morningness preference. We defined morning types as those with a score ≥ 18, and evening types as those with a score ≤ 11. The rMEQ has demonstrated good internal reliability and validity compared to the full Morningness-Eveningness Questionnaire.48
Alcohol Use Phenotypes
Aspects of alcohol use were determined by using the Registry questionnaire. Alcohol frequency was ascertained by asking, “How often do you have a drink containing alcohol?” Potential responses were never, monthly or less, 2-4 times a month, 2-3 times a week, and ≥ 4 times a week. Alcohol quantity was ascertained by asking, “How many drinks of alcohol do you have on a typical day when you are drinking?” Potential responses were 1 or 2, 3 or 4, 5 or 6, 7 to 9, and 10 or more. Binge drinking was ascertained by asking, “How often do you have 6 or more drinks on one occasion?” Potential responses were never, less than monthly, monthly, weekly, daily, or almost daily. Responses to each of the three alcohol-related questions were scored on a scale of 1 to 5.
Sociodemographics
Age, sex, and race were self-reported. Race was dichotomized into White and non-white (American Indian, Alaska Native, Native Hawaiian, Pacific Islander, Asian, black or African American, or other) categories. Education was ascertained by the question, “What is the highest level of school you have completed?” A total of 7 responses were possible, ranging from “eighth grade or less” to “graduate or professional degree.” The midpoint was “some college, but no degree or certificate.”
Statistical Analysis
We began by examining zygosity-specific twin pair correlations for diurnal preference and each of the 3 alcohol use phenotypes (alcohol frequency, alcohol quantity, and binge drinking). Within-trait, cross-twin correlations (e.g., the correlation between diurnal preference in Twin A and diurnal preference in Twin B) can be used to evaluate the magnitude of genetic and environmental influences on a given phenotype. Cross-trait, cross-twin correlations (e.g., the correlation between diurnal preference in Twin A and alcohol use frequency in Twin B) can be used to evaluate the contribution of genes to the association between the phenotypes.
Next, we evaluated these questions more formally by using the software program Mplus (Muthén & Muthén, 1998-2012, Los Angeles, CA) to fit quantitative genetic models. Specifically, we fit the bivariate twin model shown in Figure 1. Total variance in each of the observed phenotypes (boxes labeled “Diurnal Preference” and “Alcohol Use”) was decomposed into 3 latent factors: additive genetic influences (A), shared environmental influences (C, meaning common environmental influences that make siblings similar to one another), and non-shared environmental influences (E, meaning environmental influences that are unique to each twin, plus measurement error). The ACE components for each phenotype were standardized (mean = 0, standard deviation = 1) and the paths from these components to the phenotype were estimated. The correlation between additive genetic influences (A) in the first and second member of each twin pair was fixed at 1.0 in MZ twins and 0.5 in DZ twins, consistent with genetic theory. The correlation between common environmental (C) factors was fixed at 1.0 in all pair types, whereas the correlation between unique environmental (E) factors was fixed at 0 in all pair types. Finally, alcohol use was regressed on the ACE components of diurnal preference (labeled βa, βc, βe in Figure 1). These cross-paths estimate the extent to which genetic and environmental influences on diurnal preference also influence alcohol use. Note that the boxes labeled “Alcohol Use” in Figure 1 refer to each of the 3 alcohol use phenotypes, which were modeled individually. Previous authors have described the logic and parameterization of twin models in great detail.49 All models were estimated by using full information maximum likelihood to account for missing data from incomplete twin pairs.50 All models controlled for age, white race, and educational attainment by regressing both diurnal preference and alcohol use phenotypes on these covariates
Figure 1. Structural equation model of diurnal preference and alcohol use in adult twins.
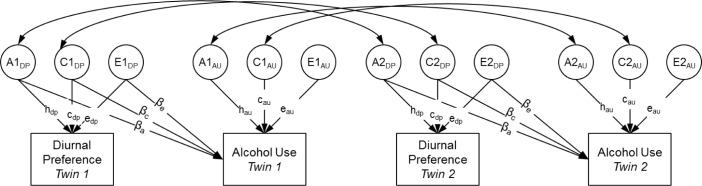
A, additive genetic variance; C, shared environmental variance; E, nonshared environmental variance. A, C, and E components standardized (mean = 0, standard deviation = 1). Correlation between A components fixed at 1.0 in monozygotic twins and 0.5 in dizygotic twins. Correlation between C components fixed to 1.0 in all twins. Correlation between E components fixed to 0 in all twins. βa, βc, and βe represent cross-paths estimating the extent to which genetic and environmental influences on diurnal preference also influence alcohol use. au, alcohol use; dp, diurnal preference.
RESULTS
Sample characteristics and descriptive statistics for all study variables are summarized in Table 1. Overall, the sample was composed of predominantly younger adults (mean = 36.4 years; standard deviation = 15.7; range 19-93) who were well-educated (64.2% with a college degree or higher) and predominantly white (88.5%) and female (64.2%). Morning types comprised 30.7% of participants, while evening types comprised 17.4%. Never drinking was reported by 21.2%. Among drinkers, 35.7% reported typically drinking ≥ 3 drinks, and 48.1% reported ≥ 1 occasion of binge drinking. Figure 2 illustrates the mean levels of alcohol frequency, alcohol quantity, and binge drinking reported by morning and evening types. Morning and evening types did not significantly differ in alcohol use frequency (p = 0.66), but evening types consumed larger quantities (p < 0.001) and were more likely to report binge drinking (p < 0.001).
Table 1.
Sample characteristics
Figure 2. Alcohol use phenotypes by morning-type versus evening-type diurnal preference.
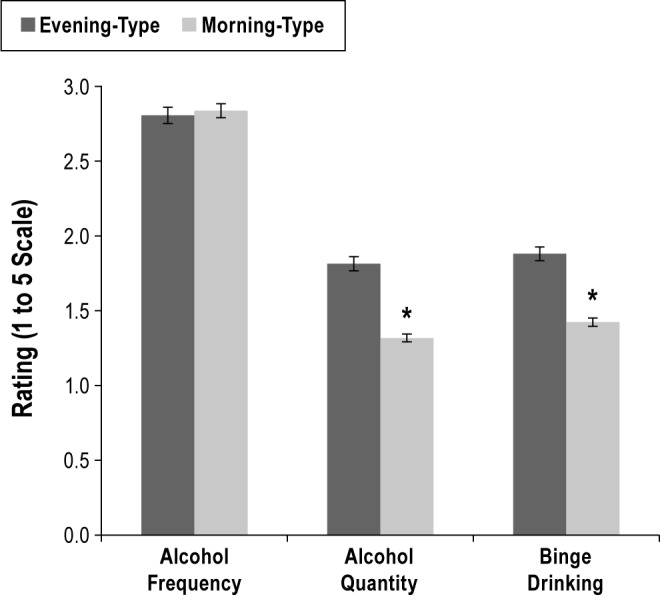
Bars represent standard error around the mean. *Significant difference.
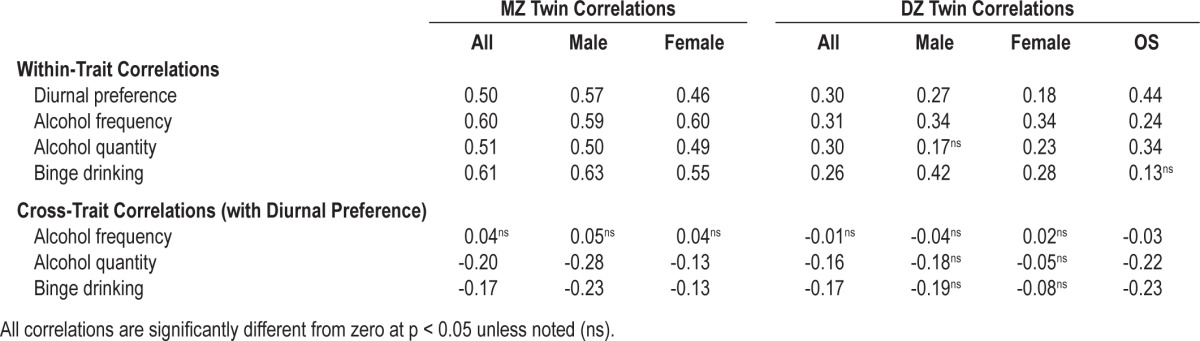
Table 2 summarizes the within-trait, cross-twin correlations for diurnal preference and alcohol use phenotypes, as well as the cross-trait, cross-twin correlations between diurnal preference and each alcohol use phenotype. Overall, the MZ correlations for each trait exceeded the DZ correlations, consistent with the presence of heritable influences on each phenotype. More specifically, descriptive heritability estimates can be calculated as h2 = 2*(rMZ – rDZ), yielding heritabilities of 40% for diurnal preference, 58% for alcohol use frequency, 42% for alcohol quantity, and 70% for binge drinking. Similarly, the cross-trait correlations (i.e., the correlation between alcohol use in Twin A and diurnal preference in Twin B) suggest that diurnal preference is more strongly related to our measures of alcohol quantity and binge drinking than to our measures of frequency of alcohol use. These initial descriptive results are formally assessed with the structural equation models.
Table 2.
Twin correlations for diurnal preference and alcohol use phenotypes
Model fit comparisons for the quantitative genetic models are summarized in Table 3. For all alcohol use outcomes, the full “ACE” model (as illustrated in Figure 1) did not fit the data significantly better than a trimmed “AE” model, in which all paths from the shared environmental factors (C) to the phenotypes (cdp, cau, and βc) were fixed at zero. In other words, shared environmental influences on alcohol use phenotypes, diurnal preference, and their associations were not significant. Consequently, we report standardized parameter estimates from the AE models in Table 4. Root mean square error of approximation (RMSEA), comparative fit index (CFI), and Tucker-Lewis Index (TLI) are alternate indices of model fit, with RMSEA values < 0.06 and CFI and TLI values > 0.95 indicating good fit.51 The overall fit for each of the 3 AE models was good (alcohol use frequency: RMSEA = 0.018, CFI = 0.99, TLI = 0.99; alcohol quantity: RMSEA = 0.020, CFI = 0.99, TLI = 0.99; binge drinking: RMSEA = 0.021, CFI = 0.99, TLI = 0.99).
Table 3.
Comparisons between quantitative genetic models
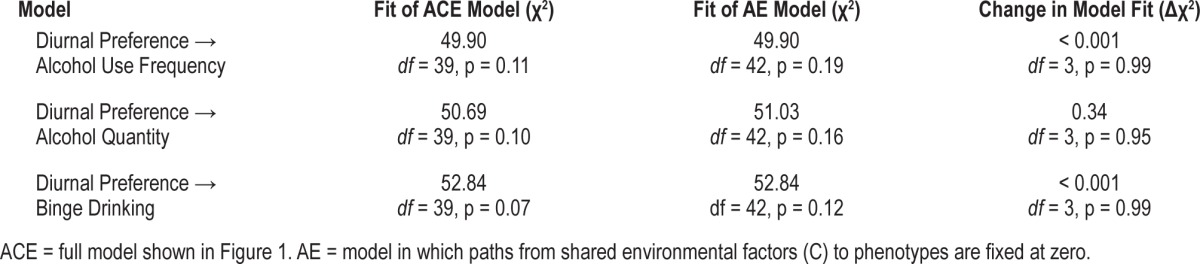
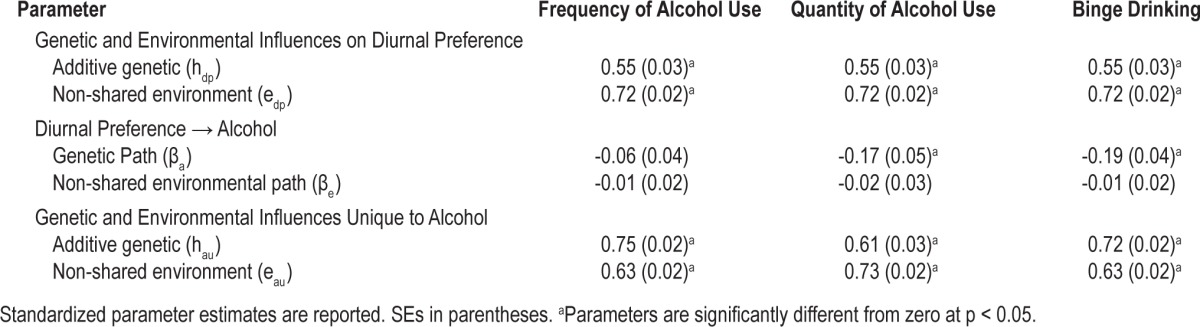
Table 4.
Results from bivariate behavioral genetic models of diurnal preference and alcohol use outcomes
Morning preference was significantly predicted by white race (β = 0.10, SE = 0.03, p < 0.05), higher educational attainment (β = 0.07, SE = 0.03, p < 0.05), and older age (β = 0.39, SE = 0.03, p < 0.05). Diurnal preference did not differ according to sex (males = 15.23, SD = 4.07; females = 15.28, SD = 3.94, p = 0.82). Alcohol use frequency, alcohol quantity, and binge drinking were not significantly associated with white race. Alcohol frequency was unrelated to age, but older people reported lower alcohol quantity (β = -0.01, SE = 0.002, p < 0.05) and less frequent binge drinking (β = -0.21, SE = 0.04, p < 0.05). People with higher educational attainment drank more frequently (β = 0.11, SE = 0.03, p < 0.05), but reported lower alcohol quantity per occasion (β = -0.124, SE = 0.03, p < 0.05). Educational attainment was not significantly associated with binge drinking.
After controlling for covariates, the proportion of residual variation in diurnal preference attributable to genetic influences can be calculated as the square of the genetic path (hdp) divided by the sum of the squared paths (hdp2 + edp2). Thus, genetic influences accounted for 37% of the variance in diurnal preference that could not be attributed to covariates, with the remaining 63% due to non-shared environmental influences. Notably, this heritability estimate is similar to that obtained in a recent study by Kuna and colleagues (41%), even though they used a different self-report instrument to assess diurnal preference.52
Genetic propensities toward eveningness were significantly associated with increased alcohol quantity (βa = -0.17; SE = 0.05, p < 0.001) and increased frequency of binge drinking (βa = -0.19; SE = 0.04, p < 0.001), but not with frequency of alcohol use. The non-shared environmental paths between diurnal preference and alcohol use phenotypes were not significant. In other words, a common set of genes influences both evening preference and elevated alcohol use, and this genetic overlap entirely accounts for the associations between diurnal preference, alcohol quantity, and binge drinking.
After accounting for variance shared with diurnal preference and with covariates, the proportions of unique variance in alcohol use frequency, alcohol quantity, and binge drinking frequency attributable to genetic influences were 59%, 41%, and 57%, respectively. The remaining variation was attributable to environmental influences unique to each twin.
DISCUSSION
We found that genetic influences on diurnal preference conferred increased risk of problematic alcohol use. Evening-type diurnal preference, alcohol quantity, and binge drinking frequency were linked by a common set of genes that entirely encompasses the association among these phenotypes. Common environmental influences were negligible, suggesting that behavior learned in early life with regard to chronotype is unrelated to familial attitudes about alcohol use—in other words, chronotype and attitudes about alcohol do not co-segregate.
Work schedules that start early in the day are most suitable for morning types. For evening types, the combination of late bedtimes driven by the endogenous clock and early waking times dictated by social factors during the work week results in short sleep and sleep debt, for which they compensate by extending sleep duration on weekends.53–55 This serves to reduce sleep quality and increase daytime sleepiness in evening types and drive associations between evening-type diurnal preference and untoward health outcomes, including psychological and psychosomatic disturbances.53,56–58 We found that evening-type twins endorsed larger quantities of alcohol consumed and more frequent binge drinking than morning-type twins, a finding consistent with previous studies.35,56,59 Alcohol consumption can represent behavioral manifestations of trouble coping with social demands,60 such as the struggles experienced by evening types who are obliged to rise early. This social situation highlights the importance of our findings for the health of evening-type twins and suggests that evening-type diurnal preference in modern society may be innately unhealthy and lead to poor health choices.
Alcohol abuse in the US exacts over $230 billion annually in costs related to crime, lost work productivity, and health-care, amounting to 2.7% of the US gross domestic product.61,62 Alcohol consumption causes 3.8% of all global deaths and is responsible for 4.6% of global disability-adjusted life-years, a composite measure of total years of healthy life lost.62 The damage to social relationships caused by alcohol abuse is harder to quantify, but no less substantial. In this context, our findings take on increased importance, as they have the potential to inform interventions to improve public and personal health. Social initiatives aimed at making work timing and other social activities more flexible for a broader range of chronotypes may reduce troublesome alcohol use. Also, elucidation of shared genetic pathways by future research may yield opportunities to develop targeted therapeutic agents that can reduce the risk of alcohol abuse in evening types.
The human circadian clock is maintained by a set of genes (CLOCK, BMAL1, PER 1, 2, and 3, CRY 1 and 2, TIM, and NPAS2) in the suprachiasmatic nucleus that control circadian rhythms, and thus diurnal preference, through a transcriptional, translational feedback loop.63 Clock genes not only control circadian rhythms, but also rhythmically regulate nearly 10,000 mammalian genes in multiple tissues involving numerous biological processes.64 NPAS2 is associated with average weekly alcohol intake,38 and polymorphisms in the CLOCK, BMAL1, PER3, and TIM genes are associated with susceptibility to mood disorders such as depression,65–68 a common risk factor for alcohol abuse.69 Polymorphisms in the serotonin transporter gene are associated with hazardous drinking in certain environmental circumstances,70 and this monoamine neurotransmitter is a key component of sleep/ wake REM/NREM brain physiology.71 Evening-type diurnal preference is linked with novelty seeking, a potential signal of reduced dopaminergic activity,39,40 while dopamine promotes wakefulness and influences sleep stages.72 These are but a few of the many potential genes and pathways that may constitute the shared genetic influences on evening-type diurnal preference and alcohol use outcomes. Future twin studies have the potential to reveal these genes and pathways by inserting polymorphisms of interest into bivariate genetic models of circadian type and alcohol use and observing the effect on the shared genetic estimates in the model.
Several issues about our study warrant discussion. Our twins were predominantly younger adult white women, and therefore our results should be applied to the general population with caution. However, this limitation is tempered by the fact our sample was derived from the community and not from a clinical population seeking healthcare, thus increasing the generalizability of our results. Subjective measures that enable the extrapolation of circadian phase, such as sleep logs, can accurately predict self-reported circadian type,73 although direct comparisons of rMEQ scores with objective measures, such as actigraphy, are lacking. Self-reported alcohol use phenotypes are, of course, subject to biases and errors in reporting; however, there is no clear alternative to self-report for measuring alcohol use in the “real world” in humans. It would be interesting for future research to examine the relation between sleep and alcohol using ecological momentary assessment technologies,74 which can yield data less subject to retrospective recall biases. Increased frequency of alcohol use was not associated with genetic propensity toward diurnal eveningness. This suggests that frequency of alcohol use represents a different aspect of alcohol consumption than the potentially more problematic constructs of increased quantity and binging which imply a lack of control of alcohol use. Lastly, diurnal preference was assigned based on a single measure, but it may represent a developmental state more than a trait. However, our analysis was age adjusted to account for this issue.
CONCLUSIONS
To our knowledge, this is the first study to show that genetic factors favoring evening-type diurnal preference confer increased risk of unhealthy phenotypes, namely binge drinking and increased alcohol consumption. This finding suggests that biological differences in circadian rhythm may be an important pathway of risk for genetic factors that promote alcohol use. It also provides further evidence that evening-type diurnal preference is an endophenotype of poor health. From a societal perspective, adjustment of school and work times to be more tolerant of evening-type diurnal preference may pay dividends at the public health level.
DISCLOSURE STATEMENT
This was not an industry supported study. This study was funded by K23 HL083350 and RC2 HL103416 from the National Heart, Lung, and Blood Institute and by a University of Washington General Clinical Research Center Pilot Grant. This work was performed at the University of Washington and the University of Texas. The authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Borbély AA. A two-process model of sleep regulation. Human Neurobiol. 1982;1:195–204. [PubMed] [Google Scholar]
- 2.Kleitman N. Sleep and wakefulness. Chicago: Chicago University Press; 1963. [Google Scholar]
- 3.Barclay NL, Gregory AM. Quantitative genetic research on sleep: A review of normal sleep, sleep disturbances and associated emotional, behavioural, and health-related difficulties. Sleep Med Rev. 2012;17:29–40. doi: 10.1016/j.smrv.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 4.Archer SN, Robilliard DL, Skene DJ, et al. A length polymorphism in the circadian clock gene per3 is linked to delayed sleep phase syndrome and extreme diurnal preference. Sleep. 2003;26:413–5. doi: 10.1093/sleep/26.4.413. [DOI] [PubMed] [Google Scholar]
- 5.Barclay NL, Eley TC, Buysse DJ, Archer SN, Gregory AM. Diurnal preference and sleep quality: Same genes? A study of young adult twins. Chronobiol Int. 2010;27:278–96. doi: 10.3109/07420521003663801. [DOI] [PubMed] [Google Scholar]
- 6.Hur Y. Stability of genetic influence on morningness—eveningness: A cross-sectional examination of South Korean twins from preadolescence to young adulthood. J Sleep Res. 2007;16:17–23. doi: 10.1111/j.1365-2869.2007.00562.x. [DOI] [PubMed] [Google Scholar]
- 7.Hur Y, Bouchard TJ, Lykken DT. Genetic and environmental influence on morningness-eveningness. Pers Individ Dif. 1998;25:917–25. [Google Scholar]
- 8.Jones KHS, Ellis J, Von Schantz M, Skene DJ, Dijk DJ, Archer SN. Age-related change in the association between a polymorphism in the per3 gene and preferred timing of sleep and waking activities. J Sleep Res. 2007;16:12–6. doi: 10.1111/j.1365-2869.2007.00561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koskenvuo M, Hublin C, Partinen M, Heikkila K, Kaprio J. Heritability of diurnal type: A nationwide study of 8753 adult twin pairs. J Sleep Res. 2007;16:156–62. doi: 10.1111/j.1365-2869.2007.00580.x. [DOI] [PubMed] [Google Scholar]
- 10.Pereira DS, Tufik S, Louzada FM. Association of the length polymorphism in the human per3 gene with the delayed sleep-phase syndrome: Does latitude have an influence upon it? Sleep. 2005:29–32. [PubMed] [Google Scholar]
- 11.Vink JM, Groot AS, Kerkhof GA, Boomsma DI. Genetic analysis of morningness and eveningness. Chronobiol Int. 2001;18:809–22. doi: 10.1081/cbi-100107516. [DOI] [PubMed] [Google Scholar]
- 12.Watson NF, Buchwald D, Noonan C, Vitiello MV, Pack AI, Goldberg J. Is circadian type associated with sleep duration in twins? Sleep Biol Rhythms. 2012;10:61–8. [Google Scholar]
- 13.Portaluppi F, Tiseo R, Smolensky MH, Hermida RC, Ayala DE, Fabbian F. Circadian rhythms and cardiovascular health. Sleep Med Rev. 2012;16:151–66. doi: 10.1016/j.smrv.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 14.Puttonen S, Harma M, Hublin C. Shift work and cardiovascular disease - pathways from circadian stress to morbidity. Scand J Work Environ Health. 2010;36:96–108. doi: 10.5271/sjweh.2894. [DOI] [PubMed] [Google Scholar]
- 15.Vyas MV, Garg AX, Iansavichus AV, et al. Shift work and vascular events: Systematic review and meta-analysis. BMJ. 2012;345:e4800. doi: 10.1136/bmj.e4800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Menegaux F, Truong T, Anger A, et al. Night work and breast cancer: A population-based case-control study in france (the CECILE study) Int J Cancer. 2012;132:924–31. doi: 10.1002/ijc.27669. [DOI] [PubMed] [Google Scholar]
- 17.Monsees GM, Kraft P, Hankinson SE, Hunter DJ, Schernhammer ES. Circadian genes and breast cancer susceptibility in rotating shift workers. Int J Cancer. 2012;131:2547–52. doi: 10.1002/ijc.27564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Savvidis C, Koutsilieris M. Circadian rhythm disruption in cancer biology. Mol Med. 2012;18:1249–60. doi: 10.2119/molmed.2012.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bonzini M, Palmer KT, Coggon D, Carugno M, Cromi A, Ferrario MM. Shift work and pregnancy outcomes: A systematic review with meta-analysis of currently available epidemiological studies. BJOG. 2011;118:1429–37. doi: 10.1111/j.1471-0528.2011.03066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rocheleau CM, Lawson CC, Whelan EA, Rich-Edwards JW. Shift work and adverse pregnancy outcomes: Comments on a recent meta-analysis. BJOG. 2012;119:378. doi: 10.1111/j.1471-0528.2011.03211.x. author reply 379–80. [DOI] [PubMed] [Google Scholar]
- 21.Kanerva N, Kronholm E, Partonen T, et al. Tendency toward eveningness is associated with unhealthy dietary habits. Chronobiol Int. 2012;29:920–7. doi: 10.3109/07420528.2012.699128. [DOI] [PubMed] [Google Scholar]
- 22.Kitamura S, Hida A, Watanabe M, et al. Evening preference is related to the incidence of depressive states independent of sleep-wake conditions. Chronobiol Int. 2010;27:1797–812. doi: 10.3109/07420528.2010.516705. [DOI] [PubMed] [Google Scholar]
- 23.Delgado Prieto P, Diaz-Morales JF, Escribano BC, Collado Mateo MJ, Randler C. Morningness-eveningness and health-related quality of life among adolescents. Span J Psychol. 2012;15:613–23. doi: 10.5209/rev_sjop.2012.v15.n2.38872. [DOI] [PubMed] [Google Scholar]
- 24.Ayas NT, White DP, Al-Delaimy WK, et al. A prospective study of self-reported sleep duration and incident diabetes in women. Diabetes Care. 2003;26:380–4. doi: 10.2337/diacare.26.2.380. [DOI] [PubMed] [Google Scholar]
- 25.Ayas NT, White DP, Manson JE, et al. A prospective study of sleep duration and coronary heart disease in women. Arch Intern Med. 2003;163:205–9. doi: 10.1001/archinte.163.2.205. [DOI] [PubMed] [Google Scholar]
- 26.Eguchi K, Hoshide S, Ishikawa S, Shimada K, Kario K. Short sleep duration is an independent predictor of stroke events in elderly hypertensive patients. J Am Soc Hypertens. 2010;4:255–62. doi: 10.1016/j.jash.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 27.Gangwisch JE, Heymsfield SB, Boden-Albala B, et al. Short sleep duration as a risk factor for hypertension: Analyses of the first national health and nutrition examination survey. Hypertension. 2006;47:833–9. doi: 10.1161/01.HYP.0000217362.34748.e0. [DOI] [PubMed] [Google Scholar]
- 28.Gangwisch JE, Heymsfield SB, Boden-Albala B, et al. Sleep duration as a risk factor for diabetes incidence in a large U.S. Sample. Sleep. 2007;30:1667–73. doi: 10.1093/sleep/30.12.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gottlieb DJ, Redline S, Nieto FJ, et al. Association of usual sleep duration with hypertension: The sleep heart health study. Sleep. 2006;29:1009–14. doi: 10.1093/sleep/29.8.1009. [DOI] [PubMed] [Google Scholar]
- 30.Hublin C, Partinen M, Koskenvuo M, Kaprio J. Sleep and mortality: A population-based 22-year follow-up study. Sleep. 2007;30:1245–53. doi: 10.1093/sleep/30.10.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kripke DF, Garfinkel L, Wingard DL, Klauber MR, Marler MR. Mortality associated with sleep duration and insomnia. Arch Gen Psychiatry. 2002;59:131–6. doi: 10.1001/archpsyc.59.2.131. [DOI] [PubMed] [Google Scholar]
- 32.Mullington JM, Simpson NS, Meier-Ewert HK, Haack M. Sleep loss and inflammation. Best Pract Res Clin Endocrinol Metab. 2010;24:775–84. doi: 10.1016/j.beem.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Watson NF, Buchwald D, Vitiello MV, Noonan C, Goldberg J. A twin study of sleep duration and body mass index. J Clin Sleep Med. 2010;6:11–7. [PMC free article] [PubMed] [Google Scholar]
- 34.Zizi F, Jean-Louis G, Brown CD, Ogedegbe G, Boutin-Foster C, McFarlane SI. Sleep duration and the risk of diabetes mellitus: Epidemiologic evidence and pathophysiologic insights. Curr Diab Rep. 2010;10:43–7. doi: 10.1007/s11892-009-0082-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Adan A. Chronotype and personality factors in the daily consumption of alcohol and psychostimulants. Addiction. 1994;89:455–62. doi: 10.1111/j.1360-0443.1994.tb00926.x. [DOI] [PubMed] [Google Scholar]
- 36.Onyper SV, Thacher PV, Gilbert JW, Gradess SG. Class start times, sleep, and academic performance in college: A path analysis. Chronobiol Int. 2012;29:318–35. doi: 10.3109/07420528.2012.655868. [DOI] [PubMed] [Google Scholar]
- 37.Urban R, Magyarodi T, Rigo A. Morningness-eveningness, chronotypes and health-impairing behaviors in adolescents. Chronobiol Int. 2011;28:238–47. doi: 10.3109/07420528.2010.549599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gamble KL, Motsinger-Reif AA, Hida A, et al. Shift work in nurses: Contribution of phenotypes and genotypes to adaptation. PLoS One. 2011;6:e18395. doi: 10.1371/journal.pone.0018395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gurpegui M, Jurado D, Luna JD, Fernandez-Molina C, Moreno-Abril O, Galvez R. Personality traits associated with caffeine intake and smoking. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:997–1005. doi: 10.1016/j.pnpbp.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 40.Hsu CY, Gau SS, Shang CY, Chiu YN, Lee MB. Associations between chronotypes, psychopathology, and personality among incoming college students. Chronobiol Int. 2012;29:491–501. doi: 10.3109/07420528.2012.668995. [DOI] [PubMed] [Google Scholar]
- 41.Le Bon O, Basiaux P, Streel E, et al. Personality profile and drug of choice; a multivariate analysis using cloninger's tci on heroin addicts, alcoholics, and a random population group. Drug Alcohol Depend. 2004;73:175–82. doi: 10.1016/j.drugalcdep.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 42.Torgersen S. The determination of twin zygosity by means of a mailed questionnaire. Acta Genet Med Gemellol (Roma) 1979;28:225–36. doi: 10.1017/s0001566000009077. [DOI] [PubMed] [Google Scholar]
- 43.Eisen S, Neuman R, Goldberg J, Rice J, True W. Determining zygosity in the vietnam era twin registry: An approach using questionnaires. Clin Genet. 1989;35:423–32. doi: 10.1111/j.1399-0004.1989.tb02967.x. [DOI] [PubMed] [Google Scholar]
- 44.Watson NF, Goldberg J, Arguelles L, Buchwald D. Genetic and environmental influences on insomnia, daytime sleepiness, and obesity in twins. Sleep. 2006;29:645–9. doi: 10.1093/sleep/29.5.645. [DOI] [PubMed] [Google Scholar]
- 45.Strachan E, Hunt C, Afari N, et al. University of Washington Twin Registry: Poised for the next generation of twin research. Twin Res Hum Genet. 2013;16:455–62. doi: 10.1017/thg.2012.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Adan A, Almirall H. Horne and östberg morningness-eveningness questionnaire: A reduced scale. Pers Individ Dif. 1991;12:241–53. [Google Scholar]
- 47.Horne JA, Östberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol. 1976;4:97–110. [PubMed] [Google Scholar]
- 48.Chelminski I, Petros TV, Plaud JJ, Ferraro R. Psychometric properties of the reduced horne and ostberg questaionnaire. Pers Individ Dif. 2000;29:469–78. [Google Scholar]
- 49.Neale MC, Maes HHM. Methodology for genetics studies of twins and families. Dordrecht, The Netherlands: Kluwer Academic; 2004. [Google Scholar]
- 50.Schafer JL, Graham JW. Missing data: Our view of the state of the art. Psychol Methods. 2002;7:147. [PubMed] [Google Scholar]
- 51.Hu LT, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Struct Equ Modeling. 1999;6:1–55. [Google Scholar]
- 52.Kuna ST, Maislin G, Pack FM, Staley B, Hachadoorian R, Coccaro EF, Pack AI. Heritability of performance deficit accumulation during acute sleep deprivation in twins. Sleep. 2012;35:1223–33. doi: 10.5665/sleep.2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Giannotti F, Cortesi F, Sebastiani T, Ottaviano S. Circadian preference, sleep and daytime behaviour in adolescence. J Sleep Res. 2002;11:191–9. doi: 10.1046/j.1365-2869.2002.00302.x. [DOI] [PubMed] [Google Scholar]
- 54.Roenneberg T, Wirz-Justice A, Merrow M. Life between clocks: Daily temporal patterns of human chronotypes. J Biol Rhythms. 2003;18:80–90. doi: 10.1177/0748730402239679. [DOI] [PubMed] [Google Scholar]
- 55.Taillard J, Philip P, Coste O, Sagaspe P, Bioulac B. The circadian and homeostatic modulation of sleep pressure during wakefulness differs between morning and evening chronotypes. J Sleep Res. 2003;12:275–82. doi: 10.1046/j.0962-1105.2003.00369.x. [DOI] [PubMed] [Google Scholar]
- 56.Mecacci L, Rocchetti G. Morning and evening types: Stress-related personality aspects. Personal Individ Diff. 1998;25:537–42. [Google Scholar]
- 57.Chelminski I, Ferraro FR, Petros TV, Plaud JJ. An analysis of the “eveningnessmorningness” dimension in “depressive” college students. J Affect Disord. 1999;52:19–29. doi: 10.1016/s0165-0327(98)00051-2. [DOI] [PubMed] [Google Scholar]
- 58.Takeuchi H, Morisane H, Iwanaga A, Hino N, Matsuoka A, Harada T. Morningness-eveningness preference and mood in japanese junior high school students. Psychiatry Clin Neurosci. 2002;56:227–8. doi: 10.1046/j.1440-1819.2002.00985.x. [DOI] [PubMed] [Google Scholar]
- 59.Taillard J, Philip P, Bioulac B. Morningness/eveningness and the need for sleep. J Sleep Res. 1999;8:291–5. doi: 10.1046/j.1365-2869.1999.00176.x. [DOI] [PubMed] [Google Scholar]
- 60.Steinhausen HC, Metzke CW. Frequency and correlates of substance use among preadolescents and adolescents in a swiss epidemiological study. J Child Psychol Psychiatry. 1998;39:387–97. [PubMed] [Google Scholar]
- 61.National Institute on Drug Abuse. [Accessed 3/11/2013]. http://www.drugabuse.gov/related-topics/trends-statistics#costs.
- 62.Rehm J, Mathers C, Popova S, Thavorncharoensap M, Teerawattananon Y, Patra J. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet. 2009;373:2223–33. doi: 10.1016/S0140-6736(09)60746-7. [DOI] [PubMed] [Google Scholar]
- 63.Albrecht U. Invited review: Regulation of mammalian circadian clock genes. J Appl Physiol. 2002;92:1348–55. doi: 10.1152/japplphysiol.00759.2001. [DOI] [PubMed] [Google Scholar]
- 64.Yan J, Wang H, Liu Y, Shao C. Analysis of gene regulatory networks in the mammalian circadian rhythm. PLoS Comput Biol. 2008;4:e1000193. doi: 10.1371/journal.pcbi.1000193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mendlewicz J. Disruption of the circadian timing systems: Molecular mechanisms in mood disorders. CNS Drugs. 2009;23(Suppl 2):15–26. doi: 10.2165/11318630-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 66.Artioli P, Lorenzi C, Pirovano A, Serretti A, Benedetti F, Catalano M, Smeraldi E. How do genes exert their role? Period 3 gene variants and possible influences on mood disorder phenotypes. Eur Neuropsychopharmacol. 2007;17:587–94. doi: 10.1016/j.euroneuro.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 67.Partonen T, Treutlein J, Alpman A, et al. Three circadian clock genes per2, arntl, and npas2 contribute to winter depression. Ann Med. 2007;39:229–38. doi: 10.1080/07853890701278795. [DOI] [PubMed] [Google Scholar]
- 68.Takao T, Tachikawa H, Kawanishi Y, Mizukami K, Asada T. Clock gene t3111c polymorphism is associated with japanese schizophrenics: A preliminary study. Eur Neuropsychopharmacol. 2007;17:273–6. doi: 10.1016/j.euroneuro.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 69.Pettinati HM, O'Brien CP, Dundon WD. Current status of co-occurring mood and substance use disorders: A new therapeutic target. Am J Psychiatry. 2013;170:23–30. doi: 10.1176/appi.ajp.2012.12010112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Laucht M, Treutlein J, Schmid B, et al. Impact of psychosocial adversity on alcohol intake in young adults: Moderation by the ll genotype of the serotonin transporter polymorphism. Biol Psychiatry. 2009;66:102–9. doi: 10.1016/j.biopsych.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 71.Monti JM. Serotonin control of sleep-wake behavior. Sleep Med Rev. 2011;15:269–81. doi: 10.1016/j.smrv.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 72.Lu BS, Zee PC. Neurobiology of sleep. Clin Chest Med. 2010;31:309–18. doi: 10.1016/j.ccm.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 73.Martin SK, Eastman CI. Sleep logs of young adults with self-selected sleep times predict the dim light melatonin onset. Chronobiol Int. 2002;19:695–707. doi: 10.1081/cbi-120006080. [DOI] [PubMed] [Google Scholar]
- 74.Shiffman S, Stone AA, Hufford MR. Ecological momentary assessment. Annu Rev Clin Psychol. 2008;4:1–32. doi: 10.1146/annurev.clinpsy.3.022806.091415. [DOI] [PubMed] [Google Scholar]


