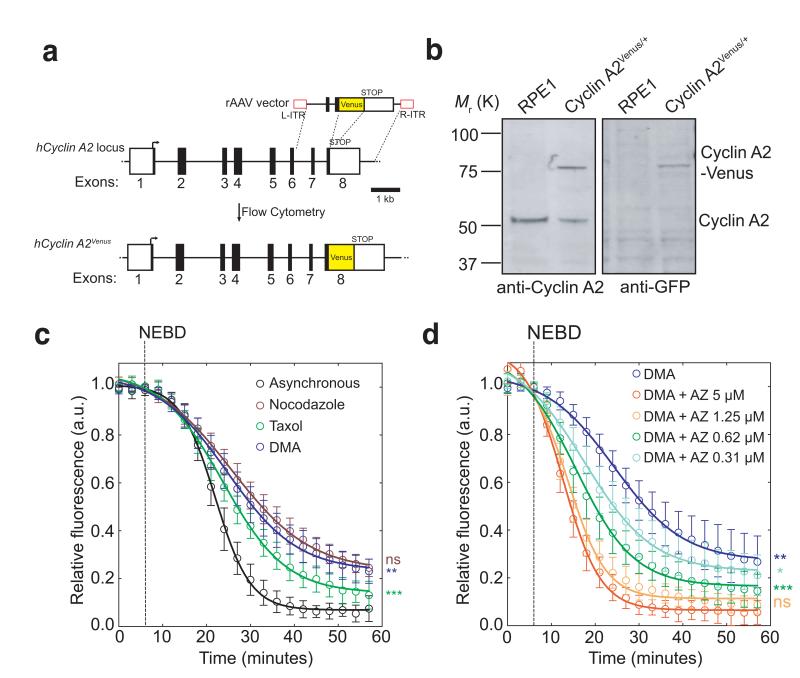
Fig. 1. Cyclin A2-Venus degradation as a readout of SAC activity.
(a), Venus ORF targeting into the hCCNA2 locus by rAAV-mediated homologous recombination. The rAAV vector contains the coding sequence of Venus, which was inserted between two gene-specific homology arms to replace the STOP codon at the junction between non-coding (white square) and coding (black square) regions in exon 8 and two inverted terminal repeats (L-ITR, R-ITR; red rectangles). Integrants were selected by fluorescence-activated cell sorting (FACS). (b), Western blot analysis of parental RPE1 and a Cyclin A2-Venus clone. Cell lysates were probed with anti-Cyclin A2 (left panel) and anti-GFP (right panel) antibodies. Note the two forms of Cyclin A2 in the Cyclin A2-Venus clone (the upper one uniquely recognized by the anti-GFP antibody). Molecular mass markers on the left. (c-d), Single-cell Cyclin A2-Venus destruction assays of asynchronously growing RPE1 Cyclin A2-Venus cells treated with nocodazole (n=15 cells), Taxol (n=12 cells) or DMA (n=12 cells) c) or different concentrations of AZ3146 in presence of 10 μM DMA (n=15 cells for all conditions except 5 μM AZ3146 where n= 11 cells) d). Images were acquired at 3-5 min intervals and the total cell fluorescence was quantified. Fluorescence intensities were normalized to the level at nuclear envelope breakdown (NEBD). Error bars indicate s.d. and are representative of at least two independent experiments. The time to reach half-maximum fluorescence after NEBD was used for statistical tests.