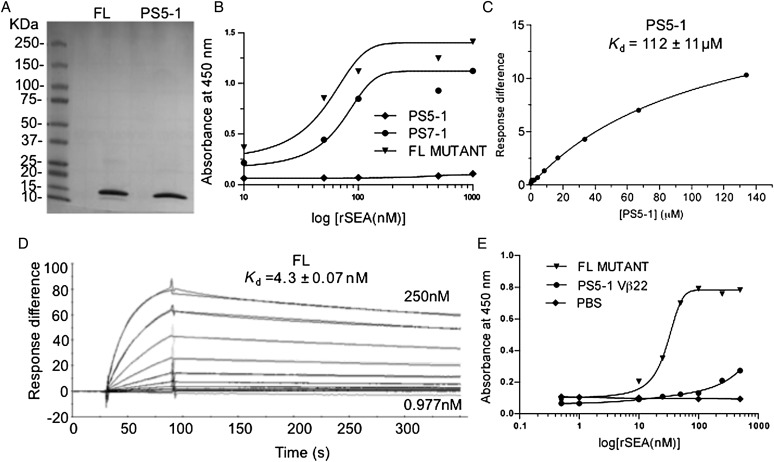
Fig. 5.
Binding analysis of soluble Vβ22 proteins. Stabilized Vβ22 (PS5-1) and high-affinity mutant (FL) were refolded from E.coli inclusion bodies and purified by Ni-NTA affinity chromatography and size-exclusion chromatography. (A) SDS–PAGE analysis of purified Vβ22 proteins. (B) ELISA-based titration for binding of immobilized Vβ22 mutant proteins to recombinant, biotinylated SEA. Purified Vβ22 mutant proteins were coated on ELISA plates at 5 μg/ml, followed by incubation with biotinylated-SEA. Bound SEA was detected using streptavidin-conjugated HRP. Data shown are representative of two or more experiments. (C) SPR-based binding analysis for the interaction between immobilized recombinant, unlabeled SEA and PS5-1 soluble protein. Non-linear regression analysis of maximal response versus concentration is depicted. (D) SPR sensorgram for the interaction between affinity matured Vβ22 mutant FL and 500 RU immobilized recombinant SEA (unlabeled). Binding affinity constants are indicated. (E) Capture ELISA to detect unlabeled SEA. Soluble Vβ22 mutant proteins were coated on the ELISA plates at 5 μg/ml. This was used to capture various concentrations of recombinant, unlabeled SEA. Bound SEA was detected with anti-SEA antibody (whole antiserum, rabbit), followed by goat anti-rabbit IgG-HRP.