Abstract
Feline cytauxzoonosis is a rapidly progressing and usually fatal disease in domestic cats caused by the tick-borne pathogen, Cytauxzoon felis. The primary reservoir host for this protozoan parasite is the bobcat (Lynx rufus). In this retrospective study, we have examined the positive cases of feline cytauxzoonosis identified at Murray State University’s Breathitt Veterinary Center, a regional diagnostic facility located in Hopkinsville, Kentucky, between January 2001 and December 2011. Center records reveal that there has been an increase in the rate of diagnosis of domestic feline infection with C. felis over that 10-year span with the majority of cases (75%) occurring between 2006–2011. The infection was diagnosed from March through October and showed a single peak in May, corresponding well with the questing period for the lone star tick, Amblyomma americanum, a known vector of C. felis.
Keywords: Cytauxzoon felis, Cytauxzoonosis
Cytauxzoonosis is a tick-borne disease caused by the Apicomplexan protozoan, Cytauxzoon felis. The first reported case in a domestic cat in the United States occurred in Missouri in 1973 (Wagner, 1976). Since then, it has been found commonly throughout the southeastern and south central United States (Birkenheuer et al., 2006; Snider et al, 2010). In recent years the geographic range of the reported feline disease has expanded further into the Mid-Atlantic and Mid-Central states (Birkenheuer et al., 2006). Jackson and Fisher (2006) published details of the first fatal case of cytauxzoonosis in eastern Kentucky in 2006; however, cases of fatal cytauxzoonosis from the western portion of the state were diagnosed at Murray State University’s Breathitt Veterinary Center as early as 2001.
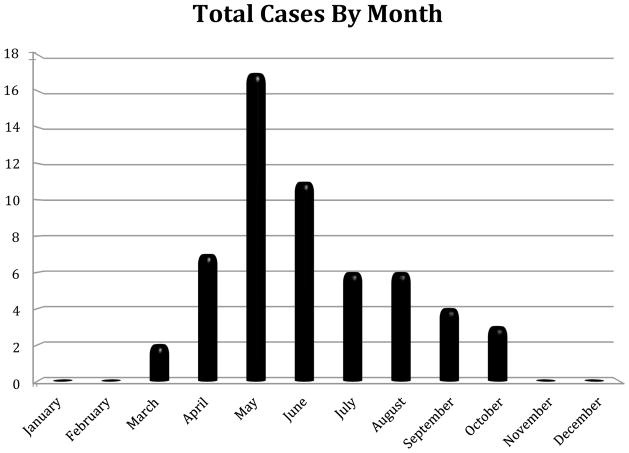
The primary vector for C. felis is still in question, as both Dermacentor variabilis (Blouin et al., 1984) and Amblyomma americanum (Reichard et al., 2010) have been shown to be viable vectors for C. felis in experimental studies. On the other hand, field studies consistently lend substantiation to the hypothesis that A. americanum is the primary vector for C. felis (Shock et al., 2011). Only wild-caught A. americanum ticks, not D. variabilis, have ever tested positive, via PCR methods, for the presence of C. felis (Reichard et al, 2010; Shock et al., 2011). In western Kentucky, D. variabilis displays two peaks in host-seeking activity – one in May/June and a second in August/September (Burg, 2001). A. americanum is most active from April until June, and then host seeking declines sharply in the heat of summer (Childs and Paddock, 2003). The data collected in the present survey demonstrate a single peak in May, with cases observed from March through October (Fig. 2), lending support for A. americanum being the potential primary vector in this area. While the presence of D. variabilis in a geographic region has shown a positive association with an increase in the number of reported cases of C. felis infection, its presence has much less of an impact than that of A. americanum (Shock et al., 2011).
FIGURE 2.
Monthly totals of cases of feline cytauxzoonosis diagnosed at the Breathitt Veterinary Center (Hopkinsville, KY) from 2001–2011.
C. felis infects a variety of wild felids, but its primary reservoir host is the bobcat, Lynx rufus. Bobcats typically develop a non-fatal, chronic parasitemia, remaining lifelong carriers of the parasite (Birkenheuer et al., 2006; Reichard et al., 2010; Jackson and Fisher, 2006). In contrast, disease in the domestic cat typically follows a very rapid sequence of events: fever, icterus, dyspnea, anorexia, lethargy, and sudden death due to severe vascular occlusion from the swelling of infected macrophages lining the vessels (Birkenheuer et al., 2006). This macrophage enlargement occurs as the C. felis schizonts undergo asexual replication (Snider et al., 2010). Subsequent rupturing of the enlarged macrophages releases merozoites, which invade erythrocytes, leading to the intraerythrocytic phase of C. felis (Wagner, 1976). This phase can result in hemolytic anemia in the cat due to rupture of the weakened, infected erythrocytes (Birkenheuer et al., 2006).
Although originally considered to be 100% fatal in domestic felines, an increasing survival rate has been observed in recent years (Shock et al., 2011). Domestic cats that survive this disease show a marked improvement within 24 days post-infection (Reichard et al., 2010). It has been difficult for investigators to find a consistent correlation between treatment regimen and the increased survival rate. Some cats have survived without receiving any treatment or supportive care, leading researchers to postulate that survival may depend more on C. felis strain variation and less on treatment regimen (Brown et al., 2009; Brown et al., 2010). A search for more efficacious treatment options for affected cats is the topic of ongoing research. Cohn and co-workers (2011) have recently reported survival rates as high as 60% in cats treated with a combination of atovaquone and azithromycin during acute infection.
Cats that survive cytauxzoonosis have the potential to become persistently parasitemic, serving as potential reservoirs for the disease (Birkenheuer et al., 2006; Reichard et al., 2010). Reichard and coworkers clearly demonstrated this in their 2010 study when a domestic cat that had survived cytauxzoonosis was shown to be capable of passing the infection to naïve cats using A. americanum as the tick vector (Reichard et al., 2010). A study of healthy, free-roaming domestic cats in Florida, North Carolina and Tennessee reported that of 961 felines captured, 0.3% tested positive for C. felis by PCR amplification (Haber et al., 2007), while a real-time PCR survey of blood samples from asymptomatic cats in Arkansas and Georgia revealed an overall prevalence of 30.3% in the 89 trapped for that study (Brown et al., 2010). More accurate testing techniques employed in the second study may have contributed to the seeming increase in reported prevalence. To our knowledge, similar surveys of C. felis infection in domestic felines have not been performed in the state of Kentucky. However, Shock and co-workers (2011) have reported an overall prevalence of 55% for C. felis infection in Kentucky bobcats (Shock et al., 2011). This is above the average for the other states tested, but below the rate of 79% for Missouri where C. felis was first described (Shock et al., 2011). The large population of both positive bobcats and increasing numbers of surviving domestic cats in Kentucky should alert all practitioners to keep C. felis as a potential diagnosis in acutely ill cats with a possible tick exposure, especially in light of the results of this study.
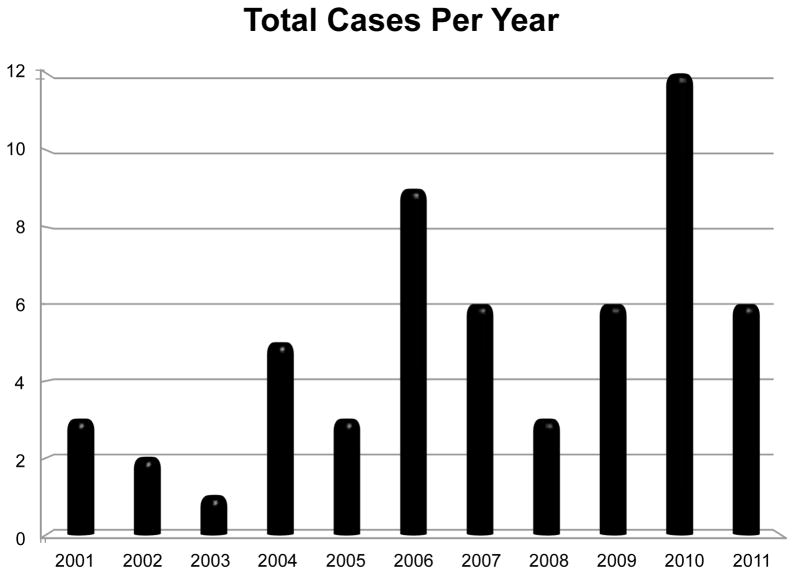
The current retrospective study of cases of C. felis infection in domestic felines utilized a database record search at Murray State University’s Breathitt Veterinary Center, a diagnostic facility located in Hopkinsville that serves western Kentucky and surrounding counties. According to facility records, which were electronically archived through 2000, a total of 56 positive cases of C. felis were diagnosed by evidence of compatible organisms in tissue samples (39%) or blood smear review (61%) at the Breathitt Veterinary Center between January 2001 and December 2011. The number of cases ranged from a low of one case in 2003 to a high of 12 cases in 2010 (see Figure 1). The majority of cases (75%) occurred from 2006 to 2011. Cytauxzoonosis was diagnosed at the center from March through October, with a single peak in May (see Figure 2). In addition, cases were mapped to county using the address of the submitting veterinarian. The highest number of cases was found in Butler County with 10, followed by Christian County with 8 (see Figure 3).
FIGURE 1.
Total number of cases of feline cytauxzoonosis diagnosed each year (2001–2011) at the Breathitt Veterinary Center (Hopkinsville, KY).
FIGURE 3.
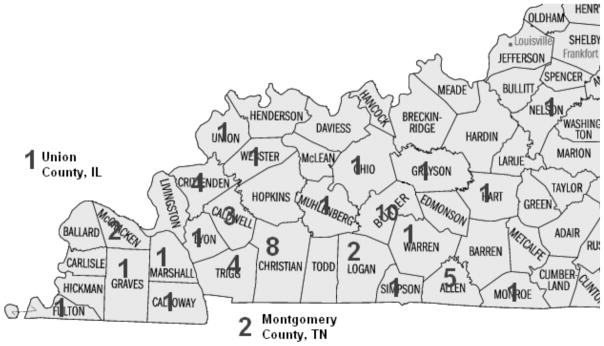
Map of feline cytauxzoonosis cases by county (county of submitting veterinarian) diagnosed at the Breathitt Veterinary Center (Hopkinsville, KY) from 2001 – 2011.
According to Dr. Lucky Pittman, Head of the Clinical Pathology Department at the Murray State University’s Breathitt Veterinary Center, criteria for positive diagnosis of C. felis begins with a thorough clinical history, taking time of year and potential tick exposure into consideration. Submitting veterinarians generally report depression, subnormal temperature, dehydration and a rapid course of disease. Necropsy findings can include dehydration, icteric mucous membranes, increased straw colored fluid in thoracic and pericardial cavities, congested and edematous lungs, and splenomegaly. Pathologists will routinely collect a full complement of tissue samples, including heart, lung, liver, kidney, spleen, stomach, small intestine, and occasionally brain. In positive cases, virtually every tissue contains macrophages full of basophilic schizonts (Figure 4) in affected animals. When a more expedient diagnosis is needed, a splenic impression smear is used to find the characteristic schizont-laden macrophages. Those samples identified in this study as positive for C. felis using only a peripheral blood smear contained numerous piroplasms in erythrocytes (Figure 5).
FIGURE 4.
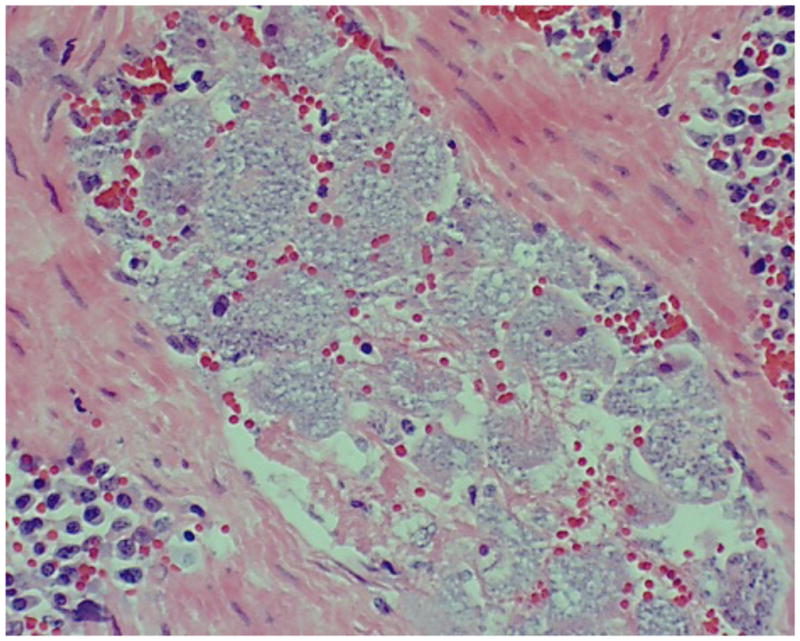
Numerous schizont-laden macrophages clearly visible in a splenic blood vessel. Tissue section from a positive case of feline cytauxzoonosis diagnosed at the Breathitt Veterinary Center (Hopkinsville, KY).
FIGURE 5.
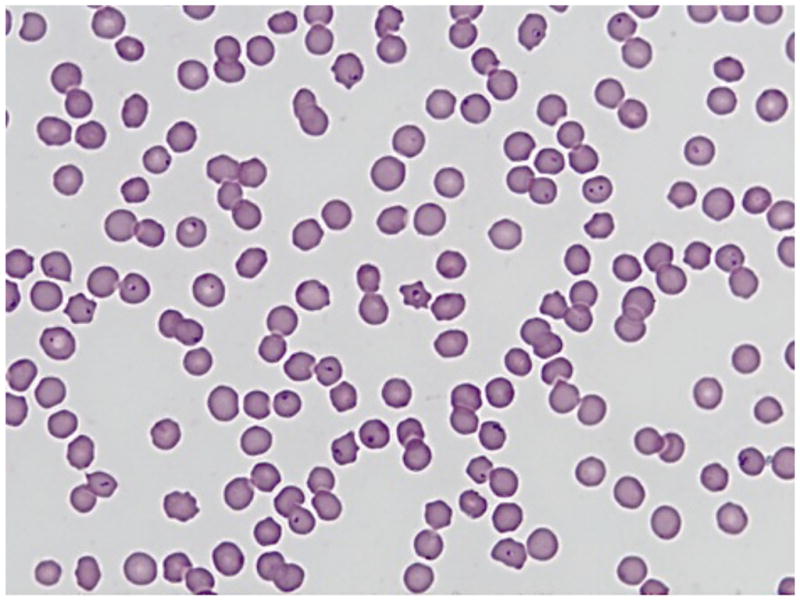
Typical erythrocytic piroplasms present in a blood smear from the first case of feline cytauxzoonosis diagnosed at the Breathitt Veterinary Center (Hopkinsville, KY) in January 2001.
There are a number of factors that could be contributing to the apparent rise in C. felis infection in domestic cats in western Kentucky. This area is highly endemic for the potential tick vectors, A. americanum and D. variabilis. There is a large deer population in the state that increases the survival rate of ticks and helps to transport them into new areas, which can potentially increase the spread of C. felis to new hosts. Population estimates for bobcats are based upon harvest data. According to the Kentucky Department of Fish and Wildlife, there has been an increase in the number killed each year from 2000 to 2011, indicating a rising population. Suitable habitats for the vectors, i.e. wooded areas with dense undergrowth, are extremely plentiful in the state. Temperature and humidity levels are more than adequate to sustain a prolonged host-seeking season. A large percentage of Kentuckians live in rural areas, giving domestic cats an increased chance of being fed upon by an infected tick. Lastly, increasing awareness by practitioners and the use of highly sensitive testing procedures by diagnosticians have led to identification of more cases of this disease.
In conclusion, the results of this retrospective study demonstrate that the disease known as feline cytauxzoonosis has been diagnosed at the Breathitt Veterinary Center in Hopkinsville, Kentucky for over 10 years. Furthermore, the frequency of diagnosed infections at the center has increased over this same time period, with 75% of cases occurring from 2006–2011. There were undoubtedly many additional cases of feline cytauxzoonosis that occurred in the state of Kentucky between 2001–2011 that were not referred to the Breathitt Veterinary Center for diagnosis. Many veterinarians diagnose the infection in-house, samples may be submitted to other diagnostic facilities, and most importantly, the majority of cats that acquire the infection are likely to go untreated.
Acknowledgments
CDD gratefully acknowledges administrative support from the National Institute of General Medical Sciences of the National Institutes of Health under award number 5P20GM103436-13.
Footnotes
Conflict of interest statement
We certify that there is no conflict of interest with any organization or person regarding this study or the information presented in this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Birkenheuer AJ, Le JA, Valenzisi AM, Tucker MD, Levy MG, Breitschwerdt EB. Cytauxzoon felis infection in cats in the Mid-Atlantic States: 34 cases (1998–2004) J Am Vet Med Assoc. 2006;228:568–571. doi: 10.2460/javma.228.4.568. [DOI] [PubMed] [Google Scholar]
- Blouin EF, Kocan AA, Glenn BL, Kocan KM, Hair JA. Transmission of Cytauxzoon felis Kier, 1979 from bobcats, Felis rufus (Schreber), to domestic cats by Dermacentor variabilis (Say) J Wildl Dis. 1984;20:241–242. doi: 10.7589/0090-3558-20.3.241. [DOI] [PubMed] [Google Scholar]
- Brown HM, Berghaus RD, Latimer KS, Britt JO, Rakich PM, Peterson DS. Genetic variability of Cytauxzoon felis from 88 infected domestic cats in Arkansas and Georgia. J Vet Diag Invest. 2009;21:59–63. doi: 10.1177/104063870902100109. [DOI] [PubMed] [Google Scholar]
- Brown HM, Lockhart JM, Latimer KS, Peterson DS. Identification and genetic characterization of Cytauxzoon felis in asymptomatic domestic cats and bobcats. Vet Parasitol. 2010;172:311–316. doi: 10.1016/j.vetpar.2010.04.041. [DOI] [PubMed] [Google Scholar]
- Burg JG. Seasonal activity and spatial distribution of host-seeking adults of the tick Dermacentor variabilis. Med Vet Entomol. 2001;15:413–21. doi: 10.1046/j.0269-283x.2001.00329.x. [DOI] [PubMed] [Google Scholar]
- Childs JE, Paddock CD. The ascendancy of Amblyomma americanum as a vector of pathogens affecting humans in the United States. Annu Rev of Entomol. 2003;48:307–337. doi: 10.1146/annurev.ento.48.091801.112728. [DOI] [PubMed] [Google Scholar]
- Cohn LA, Birkenheuer AJ, Brunker JD, Ratcliff ER, Craig AW. Efficacy of atovaquone and azithromycin or imidocarb dipropionate in cats with acute cytauxzoonosis. J Vet Intern Med. 2011;25(1):55–60. doi: 10.1111/j.1939-1676.2010.0646.x. [DOI] [PubMed] [Google Scholar]
- Haber MD, Tucker MD, Marr HS, Levy JK, Burgess J, Lappin MR, Birkenheuer AJ. The detection of Cytauxzoon felis in apparently healthy free-roaming cats in the USA. Vet Parasitol. 2007;146:316–320. doi: 10.1016/j.vetpar.2007.02.029. [DOI] [PubMed] [Google Scholar]
- Jackson CB, Fisher T. Fatal cytauxzoonosis in a Kentucky cat (Felis domesticus) Vet Parasitol. 2006;139:192–195. doi: 10.1016/j.vetpar.2006.02.039. [DOI] [PubMed] [Google Scholar]
- Reichard MV, Edwards AC, Meinkoth JH, Snider TA, Meinkoth KR, Heinz RE, Little SE. Confirmation of Amblyomma americanum (Acari: Ixodidae) as a vector for Cytauxzoon felis (Piroplasmorida: Theileriidae) to domestic cats source. J Med Entomol. 2010;47:890–896. doi: 10.1603/me10013. [DOI] [PubMed] [Google Scholar]
- Shock BC, Murphy SM, Patton LL, Shock PM, Olfenbuttel C, Beringer J, Prange S, Grove DlM, Peek M, Butfiloski JW. Distribution and prevalence of Cytauxzoon felis in bobcats (Lynx rufus), the natural reservoir, and other wild felids in thirteen states. Vet Parasitol. 2011;175:325–330. doi: 10.1016/j.vetpar.2010.10.009. [DOI] [PubMed] [Google Scholar]
- Snider TA, Confer AW, Payton ME. Pulmonary histopathology of Cytauxzoon felis infections in the cat. Vet Pathol. 2010;47:1–5. doi: 10.1177/0300985810364527. [DOI] [PubMed] [Google Scholar]
- Wagner JE. A fatal cytauxzoonosis-like disease in cats. J Am Vet Med Assoc. 1976;168:585–588. [PubMed] [Google Scholar]