Figure 5.
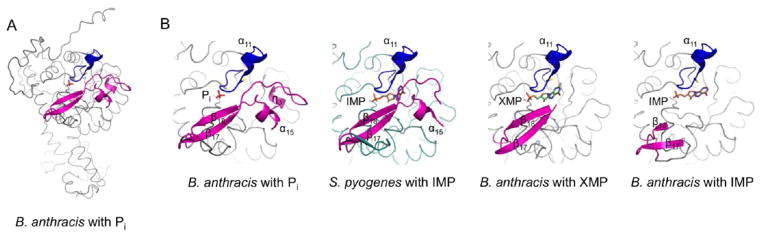
Different degrees of flap disorder in bacterial IMPDHs. (A) B. anthracis monomer (molecule A) of the highly ordered Pi-bound (apo) structure. (B) Detail of the active site, from left to right, of the B. anthracis Pi-bound enzyme (entire flap ordered), the S. pyogenes complex with IMP (missing residues 402–415 that include helix η3), and the B. anthracis complex with XMP (missing residues 394–414, including helices α15 and η3) and with IMP (missing residues 381–421), respectively. The catalytic flap is colored magenta, and the catalytic loop is colored dark blue, with the catalytic Cys represented as sticks. Ligands (Pi, IMP, and XMP) are colored red, orange, and green sticks, respectively.
