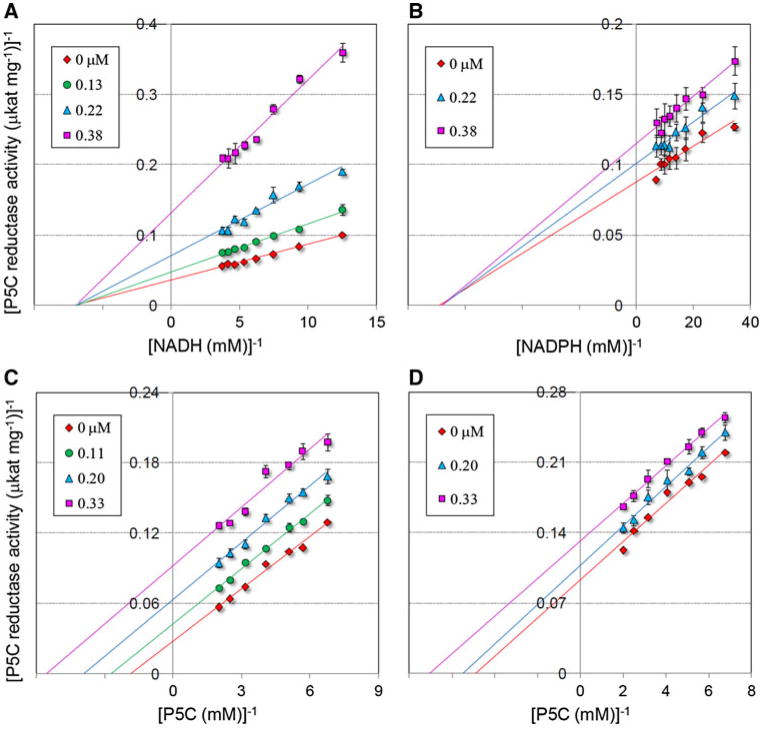
Fig. 2.
Kinetic analysis of the inhibition of S. pyogenes P5C reductase by compound 08. The enzyme was incubated with a fixed and saturating amount of P5C in the presence of increasing inhibitor concentrations, as indicated, at varying NADH (a) or NADPH (b) level. Lines converging to the x-axis in Lineweaver–Burk plot accounted for an inhibition of non competitive type. A similar analysis was carried out at varying P5C concentration, using either NADH (c) or NADPH (d) as the electron donor. Parallel lines in Lineweaver–Burk plot pointed to an inhibition of uncompetitive type. Unvariable substrate concentration was fixed at 1, 0.4 and 0.2 mM for l-P5C, NADH and NADPH, respectively