Abstract
Objective
The Medifast 5 & 1 Plan (MD) is a portion-controlled, nutritionally-balanced, low-fat weight-loss plan. We studied the effects of MD compared with a reduced-energy, food-based diet (FB) on body weight, waist circumference, fat mass, and other measures in adults.
Design
We conducted a 2 parallel-arm, randomized, controlled trial comparing MD to FB over 52 weeks. A total of 120 men and women aged 19-65 years with BMI ≥35 and ≤50 kg/m2 were randomized to MD (n = 60) or FB (n = 60). Follow-up included a 26-week weight-loss phase and 26-week weight-maintenance phase. Anthropometric, body composition, biochemical, and appetite/satiety measures were performed at baseline, 26 and 52 weeks. An intention-to-treat, linear mixed models analysis was the primary analysis.
Results
Fifty MD subjects (83.3%) and 45 FB subjects (75.0%) completed the study on assigned treatment. At 26 weeks, race-adjusted mean weight loss was 7.5 kg in MD subjects vs. 3.8 kg in FB subjects (P = 0.0002 for difference); reduction in waist circumference was 5.7 cm in MD vs. 3.7 cm in FB (P = 0.0064); and fat mass loss was 6.4 kg in MD vs. 3.7 kg in FB (P = 0.0011). At 52 weeks, the corresponding reductions were 4.7 vs. 1.9 kg (P = 0.0004); 5.0 vs. 3.6 cm (P = 0.0082); and 4.1 vs. 1.9 kg (P = 0.0019) in MD and FB subjects, respectively.
Conclusion
In obese adults, MD resulted in significantly greater reductions in body weight and fat compared with an FB diet for one year after randomization.
Keywords: Meal replacements, obesity, weight-loss diets, weight maintenance
Introduction
Obesity is increasingly prevalent and widespread in the US. In 2009-2010, more than 35% of adults were obese, and by 2010, no state had a prevalence of obesity of less than 20% (1,2). Obesity is associated with reduced lifespan (3) and many indicators of poor health (4). The causes of obesity at both the individual and population levels are manifold and are not understood completely (5-7), and many different diets have been tried to treat obesity (8). Although some degree of weight loss can be expected with all of these diets, long-term maintenance of weight loss tends to be poor (9).
One approach to address both weight loss and maintenance has been the use of portion-controlled meal-replacement diet plans. Such diets may provide a convenient alternative to controlling food intake in the current environment of ever-expanding portion sizes (10,11). The Medifast 5 & 1 Plan (MD) is a commercially available, nutritionally-balanced, low-fat meal-replacement diet. This plan includes five prepackaged meals plus a user-selected meal consisting of a lean protein source and three servings of vegetables daily.
We studied the effects of MD compared with an isoenergetic, food-based diet (FB) on multiple measures in adults, including body weight, other anthropometric measures, body composition, blood pressure, biochemical markers, and appetite and satiety measures over 52 weeks, which included weight-loss and weight-maintenance phases. We hypothesized that MD would result in greater improvements in the main outcomes – body weight, waist circumference, and fat mass – compared to FB following the weight-loss phase and less regression of these improvements following the weight-maintenance phase.
Subjects and Methods
Study design
We used a 2 parallel-arm randomized controlled trial (RCT) design. Following screening, eligible participants were randomly assigned to MD or FB. The study period was 52 weeks, and included a 26-week weight-loss phase followed by a 26-week weight-maintenance phase. Outcome measures were assessed during clinic visits at baseline and at 26 and 52 weeks after randomization. Additional contacts occurred at 2, 4, 12, 20, 28, and 46 weeks (telephone) and 8, 16, 32, and 40 weeks (clinic) to address any concerns and ask about weight loss. Non-compliant subjects were encouraged to attend the 26- and 52-week visits for assessment of main outcome measures. All visits took place between October 2010 and February 2012. The study was registered at clinicaltrials.gov (registry #NCT 01211301).
Study participants
Eligibility criteria were being generally healthy men and women aged 19 to 65 years, body mass index (BMI) between 35 and 50 kg/m2, blood pressure ≤160/95 mm Hg, fasting serum glucose level ≤126 mg/dL, and desire to lose weight. Exclusion criteria were participation in a weight-reduction program or other special diet within the previous three months; weight change of >5% of body weight in the previous six months; prior bariatric surgery or liposuction; current medications associated with suppression or stimulation of appetite; current major disease, including diabetes, cancer, uncontrolled hypertension or other cardiovascular disease, gastrointestinal disease, renal disease, chronic pulmonary disease, chronic infectious disease, or psychiatric disease; unstable doses of antidepressants, steroids, or thyroid medications; Brief Symptom Inventory 18 (12) score exceeding the 90th percentile; food allergies; history of an eating disorder or Eating Attitudes Test 40 (13) score >30; smoking within the previous six months; excessive alcohol intake; illicit drug use; pregnancy, recent childbirth, or nursing; and no usual source of health care.
Subjects were recruited from the Birmingham, AL area through an e-mailed flyer, campus newspaper advertisement, databases of persons interested in weight loss, and word of mouth. Subjects were screened for initial eligibility by telephone. Eligible subjects attended a clinic visit for further screening, during which fasting serum glucose concentration was assessed; height, weight, and blood pressure were measured; and screening questionnaires were completed. Subjects who passed this second level of screening and were interested in proceeding were randomly assigned to one of two study groups (Figure 1). The protocol was approved by the Institutional Review Board for Human Use at the University of Alabama at Birmingham (UAB), and informed consent was obtained from each subject.
Figure 1.
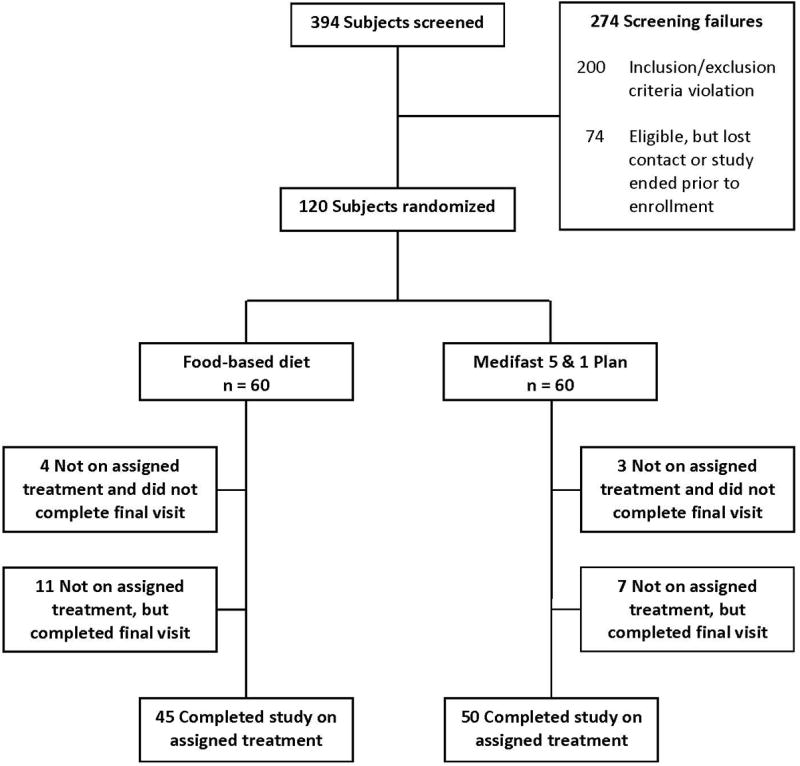
Consort diagram.
Randomization
At the baseline visit, eligible participants were randomly assigned to the MD or FB group via a pseudorandom number generator with a 1:1 allocation ratio. The allocated group was indicated on cards contained in sequentially numbered, opaque, sealed envelopes prepared in the Department of Biostatistics, UAB School of Public Health. To randomize a participant, the study coordinator opened the next consecutively numbered envelope in the presence of the participant.
Intervention
MD group: weight-loss phase
At baseline, subjects randomized to MD received a Quick Start Guide providing details of the plan and were encouraged to enroll in the MD online support “community.” MD offers services and products via multiple venues. This trial used the one offered to MD customers who purchase the company's products by phone or website. The plan consisted of five portion-controlled, nutritionally-balanced, low-fat meals plus one “Lean & Green” meal each day. MD meals came in individual packages supplied by the company (Medifast, Inc., Owings Mills, MD). More than 70 MD meal choices were used interchangeably, so that any five meals could be selected by a participant for their daily meals. MD provided approximately 800-1,000 kcal/d, depending on personal selections. The Lean & Green meal consisted of a lean protein source plus vegetables selected and provided by the participant from a list provided in the Quick Start Guide. The “Lean” portion included 5-7 ounces (cooked weight) of lean meat or meatless protein source and 0-2 servings of healthy fat (based on protein choice). The “Green” portion included any three servings of low-carbohydrate/non-starchy vegetables (1/2 cup or 1 cup depending on vegetable choices).
MD support consisted of online access to MD resources, including dietitians, trainers, recipes, message boards, and chat rooms, allowing participants to interact with others on the same plan. MD meal ordering was via telephone between the company and participant. MD participants were given a menu, ordering instructions, and a dedicated coupon code to ensure that products were sent to their homes at no charge to them. Participants ordered one month of meals and were encouraged to use the auto-ship feature to prevent inadvertently running out of meals. Participants unsatisfied with any MD product could exchange it for another at the clinic. Because MD meal replacements are fortified with vitamins and minerals, MD participants were advised to not take a daily multi-vitamin/mineral supplement.
FB group: weight-loss phase
Participants randomly assigned to the FB group were provided with a 1,000-kcal/d meal plan based on regular foods selected, procured, and prepared by the participants. Food lists, sample menus, and a portion size reference were provided. FB participants were referred to the MyPyramid.gov (currently ChooseMyPlate.gov) website for nutrition information. To ensure adequate nutrient intake, FB participants were advised to take a daily multi-vitamin/mineral supplement.
Both groups: weight-maintenance phase
During the 26-week weight-maintenance phase, we calculated estimates of energy needs for subjects in both groups to maintain weight by use of a computer-generated basal metabolic rate (BMR) based on lean body mass measured by bioelectrical impedance analysis (BIA). BMR was multiplied by an activity factor (based on self report) to calculate total energy expenditure. The MD group was given the option of including 0-3 MD meals/d to reach their energy needs for weight maintenance, whereas the FB group remained wholly food-based.
Measures
Body weight was assessed at baseline and at the 26- and 52-week clinic visits as outcome measures (and at the 8-, 16-, 32-, and 40-week clinic visits as a check of participant progress) with participants in light clothing and no shoes using a Tanita model BC-418 digital scale/body composition analyzer (Tanita Corporation of America, Inc., Arlington Heights, IL). Height was measured at baseline without shoes using a Harpenden wall-mounted stadiometer. BMI was calculated as kg/m2 using measured weight and height. Waist circumference was measured at baseline, 26 weeks, and 52 weeks with a constant-tension tape measure on bare skin at the natural indentation between the 10th rib and iliac crest at the end of a normal expiration. Body composition was assessed at baseline, 26 weeks, and 52 weeks by BIA using the scale/segmental body composition analyzer. Blood pressure was measured at the same time points with a DINAMAP ProCare 100 blood pressure monitor (GE Healthcare, Waukesha, WI). Upper arm circumference was measured to determine appropriate cuff size. Blood pressure was measured twice following a 5-minute rest period with a 30-second rest period between measurements. Analyses used the mean of the two measurements. All measurements were performed by a single staff person, trained and certified in measurement procedures.
Fasting blood samples were collected at the baseline, 26-week, and 52-week visits. Lipid (total cholesterol, low-density lipoprotein [LDL] cholesterol, high-density lipoprotein [HDL] cholesterol, and triglycerides) and hepatic function (alanine aminotransferase [ALT], aspartate aminotransferase [AST], and alkaline phosphatase) panels were performed by a clinical diagnostic laboratory (Quest Diagnostics, Madison, NJ). Glucose and high-sensitivity C-reactive protein (hs-CRP) assays were performed in the Metabolism/Human Physiology Core Laboratory at UAB. Glucose was assayed with a glucose oxidase reagent on a Sirrus analyzer (Stanbio Laboratory, Boerne, TX), with an inter-assay CV of 3.1% and a minimum sensitivity of 1 mg/dL. hs-CRP was assayed with a turbidometric method on the Sirrus analyzer, with an inter-assay CV of 2.1% and a minimum sensitivity of 0.05 mg/L. Lipid hydroperoxides (LPO) were measured in the Bioanalytical Redox Biology Core Laboratory at UAB with a ferric thiocyanate assay (Cayman Chemical Company, Ann Arbor, MI).
At the 26-week and 52-week visits, appetite sensations (including hunger, fullness, thoughts of food, urges to eat, and food cravings) were assessed with validated visual analog scales (VAS) (14). Questionnaires also were completed at these two visits to assess self-reported adherence to the respective diet plans during the weight-loss and weight-maintenance phases.
Statistical analysis
All data were screened for univariate and multivariate outliers to detect any potential misentries, and any transcription errors detected were corrected. In order to conduct intention-to-treat analyses using all randomized subjects, we performed linear mixed models with time of measurement, treatment condition (MD vs. FB), and the treatment-by-time interaction as the predictors of interest. For each outcome analyzed, the baseline measure was used as a covariate; thus, two time points (weeks 26 and 52) comprised the within-subject time factor. With two time points, a compound-symmetric covariance structure was assumed. Residuals were examined to evaluate the normality and homoscedasticity assumptions. Although there were no extreme departures from normality, we detected heterogeneity of variance across experimental groups on several outcomes. Thus, due to the change in the number of observations over time and this heterogeneity of variance, separate covariance functions were estimated, and Kenward-Roger adjustments (15) to the error degrees-of-freedom were calculated. Because this correction for heterogeneity of covariance matrices did not affect the major conclusions, we reported the results of tests assuming equal unstructured covariance structures. Secondary analyses to compute statistical tests for within-group change used linear mixed models across all three time points. Given that race has been associated with weight loss achieved in previous trials (16), and we observed modest imbalance in race between the MD and FB groups, we elected to adjust the results for race. Because this was not pre-planned, in accordance with Consolidated Standards for Reporting Trials (CONSORT) (17), we present results both adjusted and unadjusted for race.
Two sensitivity analyses were conducted. First, we performed the same set of analyses for the subset of subjects that completed the study on their assigned treatment regimen (i.e., completers only). Second, to increase comparability with other published trials (although we do not endorse the method), we used an imputed data set in which any missing data were replaced with the last observation carried forward. Analyses were performed using SAS statistical software, version 9.2 (SAS institute Inc., Cary, NC).
Results
A total of 120 persons were randomly assigned to either FB (n = 60) or MD (n = 60) (Figure 1). The majority of subjects were well-educated African-American women. There were no statistically significant differences in sex, age, race, or education between the two groups (Table 1). Fifty MD subjects (83.3%) completed the 52-week study period on the assigned treatment compared with 45 FB subjects (75.0%) (P for difference = 0.3689). Ten MD subjects and 15 FB subjects stopped following their assigned meal plan or had chosen a different weight-loss plan at some point prior to the final clinic visit at 52 weeks (designated “not on assigned treatment”). Seven of the 10 MD subjects and 11 of the 15 FB subjects not on assigned treatment did complete the final clinic visit.
Table 1. Characteristics of the study participants at baseline.
| FB (n = 60) | MD (n = 60) | P value | |
|---|---|---|---|
| Sex, n (%) | |||
| Female | 54 (90.0) | 52 (86.7) | 0.5695a |
| Male | 6 (10.0) | 8 (13.3) | |
| Age, mean (SD) | 39.7 (9.1) | 40.2 (9.2) | 0.7958b |
| Race, n (%) | |||
| White | 19 (31.7) | 9 (15.0) | 0.0827a |
| African American | 40 (66.7) | 50 (83.3) | |
| Other | 1 (1.7) | 1 (1.7) | |
| Education, n (%) | |||
| Less than high school | 1 (1.7) | 0 (0.0) | 0.6319a |
| High school diploma/GED | 2 (3.3) | 3 (5.0) | |
| Some college | 25 (41.7) | 26 (43.3) | |
| College degree | 21 (35.0) | 20 (33.3) | |
| Graduate/professional degree | 11 (18.3) | 11 (18.3) | |
Abbreviations: FB, reduced-energy, food-based diet; GED, Graduate Equivalency Degree; MD, Medifast 5 & 1 Plan.
P value based on Pearson Chi-Square test for between-group differences.
P value based on t-test for between-group differences.
Self-reported adherence to the diets generally was “fair” during both the weight-loss and weight-maintenance phases. There were no significant differences between the groups in self-reported adherence to the assigned diets at weeks 26 or 52, nor were there significant differences in changes in adherence between week 26 and week 52. The mean (SD) number of meal replacements reported over the previous 2 weeks by the MD group at week 26 was 33.3 (25.5) of the maximum 70 if the subjects had used 5 meal replacements per day for the 14 days. Also at week 26, MD subjects reported that they consumed the Lean & Green meal most days over the past 2 weeks. During the 26-week maintenance phase, MD subjects reported consuming a mean (SD) of 56.5 (83.6) meal replacements (which were optional during this phase), or about two per week (data not shown).
26 Weeks
In results adjusted for race, body weight, BMI, waist circumference, fat mass, and fat-free mass were significantly reduced in the MD group at 26 weeks compared with baseline. In the FB group, there were significant reductions in all of these measures except for fat-free mass (Table 2). In both groups, there were significant increases in fat-free mass/weight. Weight loss was greater in the MD group compared with the FB group (−7.5 vs. −3.8 kg; P = 0.0002), corresponding to a mean 6.7% loss of baseline weight in the MD group compared with a mean 3.4% loss in the FB group. There were corresponding statistically significant greater decreases in BMI (−2.6 vs. −1.4 kg/m2; P = 0.0005), waist circumference (−5.7 vs. −3.7 cm; P = 0.0064), fat mass (−6.4 vs. −3.7 kg; P = 0.0011), and fat-free mass (−1.2 vs. −0.2 kg; P = 0.0162), and increase in fat-free mass/weight (+2.9 vs. +1.9 %; P = 0.0110) in the MD group compared with the FB group. There were no significant differences between the groups in changes in systolic or diastolic blood pressure from baseline to 26 weeks.
Table 2. Anthropometric measures, body composition, and blood pressure in the two groups at baseline and follow-up: intention-to-treat analysis.
| Baseline | Week 26 | Change from baseline | Week 52 | Change from baseline | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||||
| FB (n=60) | MD (n=60) | FB (n=49) | MD (n=56) | FB (n=49) | MD (n=56) | P valuea | FB (n=56) | MD (n=57) | FB (n=56) | MD (n=57) | P valueb | |
| Weight (kg) | 113.0c | 111.9 | 109.1 | 104.4 | −3.8* | −7.5* | 0.0002 | 111.0 | 107.2 | −1.9* | −4.7* | 0.0004 |
| [112.9]d | [112.6] | [108.8] | [104.7] | [−4.2†] | [−7.6†] | [0.0115] | [111.0] | [107.7] | [−1.9†] | [−4.7] | [0.0342] | |
| (12.2)e | (16.8) | (13.8) | (16.6) | (7.1) | (8.3) | (13.0) | (16.5) | (7.0) | (7.0) | |||
| BMI (kg/m2) | 41.4 | 40.4 | 40.1 | 37.8 | −1.4* | −2.6* | 0.0005 | 40.8 | 38.8 | −0.7* | −1.6* | 0.0012 |
| [41.3] | [40.6] | [39.9] | [37.9] | [−1.5†] | [−2.6†] | [0.0147] | [40.6] | [38.9] | [−0.6†] | [−1.6†] | [0.0395] | |
| (3.8) | (3.8) | (4.6) | (4.7) | (2.4) | (2.8) | (4.5) | (4.3) | (2.4) | (2.4) | |||
| Waist (cm) | 111.4 | 107.8 | 107.6 | 102.0 | −3.7* | −5.7* | 0.0064 | 107.8 | 102.8 | −3.6* | −5.0* | 0.0082 |
| [111.5] | [107.8] | [107.4] | [101.6] | [−4.1†] | [−5.7†] | [0.0390] | [107.8] | [102.4] | [−3.5†] | [−5.0†] | [0.0692] | |
| (10.3) | (10.7) | (10.6) | (11.1) | (5.6) | (5.8) | (10.2) | (11.1) | (5.2) | (5.1) | |||
| Fat mass (kg) | 54.2 | 53.3 | 50.5 | 47.0 | −3.7* | −6.4* | 0.0011 | 52.3 | 49.2 | −1.9* | −4.1* | 0.0019 |
| [53.8] | [53.7] | [50.1] | [47.3] | [−4.0†] | [−6.5†] | [0.0253] | [51.9] | [49.5] | −1.9†] | [−4.2†] | [0.0430] | |
| (8.8) | (10.0) | (10.8) | (11.6) | (5.9) | (6.5) | (11.1) | (10.9) | (5.8) | (5.7) | |||
| Fat-free mass (kg) | 58.7 | 58.6 | 58.5 | 57.4 | −0.2 | −1.2* | 0.0162 | 58.7 | 58.0 | 0.0 | −0.6 | 0.0600 |
| [59.1] | [58.9] | [58.7] | [57.6] | [−0.2] | [−1.1†] | [0.0764] | [59.1] | [58.1] | [0.0] | [−0.5] | [0.2803] | |
| (9.1) | (11.3) | (9.4) | (10.2) | (2.9) | (3.2) | (8.6) | (10.7) | (2.9) | (2.8) | |||
| Fat-free mass/weight (%) | 52.0 | 52.4 | 54.0 | 55.3 | +1.9* | +2.9* | 0.0110 | 53.2 | 54.3 | +1.1* | +1.9* | 0.0292 |
| [52.4] | [52.3] | [54.2] | [55.3] | [+2.0†] | [+3.0†] | [0.0968] | [53.5] | [54.1] | [+1.1†] | [+1.9†] | [0.1915] | |
| (5.3) | (5.2) | (6.6) | (6.4) | (3.2) | (3.3) | (6.8) | (5.8) | (3.2) | (2.9) | |||
| SBP (mm Hg) | 124.1 | 124.0 | 120.9 | 120.9 | −3.2 | −3.2 | 0.9999 | 124.8 | 123.5 | +0.8 | −0.5 | 0.4730 |
| [124.2] | [124.2] | [121.1] | [120.8] | [−3.9] | [−3.0] | [0.9786] | [125.2] | [123.5] | [+0.8] | [−0.3] | [0.4837] | |
| (12.2) | (14.5) | (11.1) | (14.4) | (11.7) | (13.3) | (12.2) | (13.8) | (10.0) | (11.4) | |||
| DBP (mm Hg) | 73.8 | 74.7 | 73.1 | 73.0 | −0.7 | −1.6 | 0.6218 | 73.6 | 75.2 | −0.1 | +0.6 | 0.3650 |
| [73.8] | [74.9] | [72.8] | [72.7] | [−0.7] | [−1.4] | [0.6534] | [73.7] | [75.0] | [−0.2] | [+0.7] | [0.3098] | |
| (6.9) | (8.7) | (7.5) | (8.5) | (5.6) | (7.1) | (7.5) | (8.5) | (5.9) | (5.7) | |||
Abbreviations: BMI, body mass index; DBP, diastolic blood pressure; FB, reduced-energy, food-based diet; MD, Medifast 5 & 1 Plan; SBP, systolic blood pressure.
P <0.05 for within-group difference in change in measure between baseline and week 26 or baseline and week 52 from mixed model analysis of covariance with adjustment for race.
P <0.05 for within-group difference in change in measure between baseline and week 26 or baseline and week 52 from mixed model analysis.
P value for between-group difference in change in measure at week 26 from mixed model analysis of covariance with adjustment for the relevant baseline measure and race [p-value in parentheses does not adjust for race as a covariate].
P value for between-group difference in change in measure at week 52 from mixed model analysis of covariance with adjustment for the relevant baseline measure and race [p-value in parentheses does not adjust for race as a covariate].
Mean (adjusted for race).
Mean (unadjusted).
Standard deviation.
Total cholesterol, LDL cholesterol, hs-CRP, ALT, AST, and LPO were significantly decreased in the MD group at 26 weeks compared with baseline; in the FB group, there was a significant reduction in LPO (Table 3). Total and LDL cholesterol decreased to a greater degree in the MD group than in the FB group (−8.4 vs. −1.1 mg/dL; P = 0.0355 and −9.2 vs. −3.0 mg/dL; P = 0.0119, respectively). LPO decreased slightly more in the MD group than in the FB group (−3.4 vs. −2.6 μM; P = 0.0289).
Table 3. Biochemical measures in the two groups at baseline and follow-up: intention-to-treat analysis.
| Baseline | Week 26 | Change from baseline | Week 52 | Change from baseline | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||||
| FB (n=60) | MD (n=60) | FB (n=49) | MD (n=56) | FB (n=49) | MD (n=56) | P valuea | FB (n=56) | MD (n=57) | FB (n=56) | MD (n=57) | P valueb | |
| TC (mg/dL) | 183.1c | 179.7 | 182.0 | 171.3 | −1.1 | −8.4* | 0.0355 | 183.6 | 179.4 | +0.5 | −0.3 | 0.5956 |
| [182.8]d | [178.9] | [185.1] | [171.1] | [−1.0] | [−8.8†] | [0.0316] | [184.4] | [178.2] | [+0.3] | [−0.4] | [0.5949] | |
| (42.1)e | (30.3) | (32.5) | (26.0) | (32.8) | (17.7) | (37.1) | (32.1) | (34.2) | (19.5) | |||
| LDL-C (mg/dL) | 113.8 | 107.6 | 110.8 | 98.4 | −3.0 | −9.2* | 0.0119 | 110.9 | 106.4 | −2.8 | −1.3 | 0.9692 |
| [113.5] | [107.1] | [112.8] | [98.6] | [−2.3] | [−9.5†] | [0.0088] | [111.9] | [105.9] | [−2.9] | [−1.5] | [0.9472] | |
| (36.3) | (27.0) | (27.7) | (25.9) | (18.1) | (16.7) | (33.1) | (30.3) | (29.4) | (16.1) | |||
| HDL-C (mg/dL) | 50.2 | 52.3 | 51.1 | 53.5 | +0.9 | +1.2 | 0.4399 | 51.2 | 54.5 | +1.1 | +2.1 | 0.2042 |
| [49.7] | [52.9] | [52.0] | [54.5] | [+0.7] | [+1.1] | [0.5248] | [50.9] | [55.3] | [+1.0] | [+2.2] | [0.2543] | |
| (14.6) | (14.2) | (15.3) | (11.5) | (10.3) | (8.4) | (15.4) | (13.5) | (8.5) | (8.8) | |||
| TG (mg/dL) | 108.6 | 98.0 | 103.5 | 94.4 | −5.1 | −3.7 | 0.3606 | 106.7 | 90.6 | −2.0 | −7.5 | 0.0699 |
| [110.4] | [94.1] | [102.7] | [89.0] | [−5.9] | [−2.5] | [0.3742] | [108.5] | [85.1] | [−2.2] | [−5.7] | [0.0704] | |
| (57.6) | (51.3) | (47.2) | (46.9) | (43.0) | (34.9) | (53.9) | (28.9) | (58.3) | (35.8) | |||
| Glucose (mg/dL) | 102.0 | 99.0 | 104.0 | 98.0 | +2.0 | −1.1 | 0.0535 | 102.6 | 96.8 | +0.6 | −2.2 | 0.0533 |
| [102.5] | [98.6] | [103.6] | [97.5] | [+1.1] | [−1.0] | [0.0886] | [103.0] | [96.2] | [+0.6] | [−2.3] | [0.0874] | |
| (13.7) | (12.6) | (18.0) | (9.3) | (14.9) | (10.4) | (19.0) | (12.1) | (13.7) | (10.2) | |||
| hs-CRP (mg/L) | 10.4 | 9.9 | 9.7 | 7.7 | −0.7 | −2.2* | 0.0538 | 9.9 | 7.0 | −0.5 | −2.9* | 0.0021 |
| [10.1] | [10.1] | [9.6] | [8.0] | [−1.1] | [−2.2†] | [0.1082] | [9.7] | [7.2] | [−0.6] | [−2.9†] | [0.0065] | |
| (9.2) | (8.5) | (10.4) | (6.4) | (5.1) | (4.6) | (8.6) | (6.4) | (5.6) | (5.0) | |||
| ALP (U/L) | 75.6 | 78.7 | 76.2 | 77.9 | +0.6 | −0.8 | 0.3696 | 74.5 | 77.5 | −1.1 | −1.2 | 0.7988 |
| [76.2] | [78.0] | [77.0] | [78.9] | [+0.4] | [−0.6] | [0.4377] | [75.5] | [78.9] | [−1.2] | [−0.7] | [0.8893] | |
| (20.9) | (34.7) | (25.0) | (49.0) | (11.7) | (17.2) | (20.3) | (57.4) | (9.3) | (25.6) | |||
| ALT (U/L) | 18.4 | 20.5 | 16.5 | 16.7 | −1.9 | −3.8* | 0.2356 | 17.4 | 18.2 | −1.0 | −2.4 | 0.7348 |
| [18.5] | [20.5] | [16.5] | [16.7] | [−2.1] | [−3.9†] | [0.2578] | [17.8] | [18.1] | [−1.0] | [−2.4] | [0.7566] | |
| (10.8) | (16.8) | (8.3) | (11.9) | (5.5) | (9.0) | (10.7) | (20.0) | (5.8) | (12.0) | |||
| AST (U/L) | 16.6 | 18.6 | 17.0 | 17.0 | +0.4 | −1.6* | 0.1649 | 16.8 | 17.6 | +0.3 | −1.0 | 0.8563 |
| [16.7] | [18.4] | [17.0] | [16.8] | [+0.2] | [−1.7†] | [0.0765] | [17.1] | [17.4] | [+0.4] | [−1.0] | [0.6824] | |
| (5.6) | (10.8) | (4.5) | (7.6) | (4.3) | (6.1) | (6.1) | (13.9) | (4.6) | (8.5) | |||
| LPO (μM) | 7.7 | 7.7 | 5.1 | 4.3 | −2.6* | −3.4* | 0.0289 | 7.1 | 9.0 | −0.6 | +1.4 | 0.0933 |
| [7.7] | [7.7] | [5.1] | [4.2] | [−2.8†] | [−3.2†] | [0.0171] | [7.1] | [9.0] | [−0.8] | [+1.6] | [0.1032] | |
| (4.2) | (3.8) | (1.9) | (1.6) | (4.4) | (4.5) | (4.7) | (7.2) | (6.3) | (8.0) | |||
Abbreviations: ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; FB, reduced-energy, food-based diet; HDL-C, high-density lipoprotein cholesterol; hs-CRP, C-reactive protein; LDL-C, low-density lipoprotein cholesterol; LPO, lipid hydroperoxides; MD, Medifast 5 & 1 Plan; TC, total cholesterol; TG, triglycerides.
P <0.05 for within-group difference in change in measure between baseline and week 26 or baseline and week 52 from mixed model analysis of covariance with adjustment for race.
P <0.05 for within-group difference in change in measure between baseline and week 26 or baseline and week 52 from mixed model analysis.
P value for between-group difference in change in measure at week 26 from mixed model analysis of covariance with adjustment for the relevant baseline measure and race [p-value in parentheses does not adjust for race as a covariate].
P value for between-group difference in change in measure at week 52 from mixed model analysis of covariance with adjustment for the relevant baseline measure and race [p-value in parentheses does not adjust for race as a covariate].
Mean (adjusted for race).
Mean (unadjusted).
Standard deviation.
52 Weeks
At 52 weeks, in results adjusted for race, weight, BMI, waist circumference, and fat mass were significantly decreased in both the MD and FB groups compared with baseline (Table 2). Fat-free mass/weight was significantly increased in both the MD and FB in both groups at 52 weeks. The weight reduction at 52 weeks was attenuated in both groups compared with that at 26 weeks, but continued to be greater in the MD group compared with the FB group (−4.7 vs. −1.9 kg; P = 0.0004), corresponding to a mean 4.2% loss of baseline weight in the MD group compared with a mean 1.7% loss in the FB group. Reductions in BMI (−1.6 vs. −0.7 kg/m2; P = 0.0012), waist circumference (−5.0 vs. −3.6 cm; P = 0.0082), fat mass (−4.1 vs. −1.9 kg; P = 0.0019), and increase in fat-free mass/weight (+1.9 vs. +1.1 %; P = 0.0292) were attenuated in both groups compared with those at 26 weeks, but remained significantly greater in the MD group.
Also at 52 weeks, hs-CRP was significantly reduced compared with baseline in the MD group, whereas there were no significant reductions in the FB group (Table 3). Of the biochemical measures, only the reduction in hs-CRP was significantly greater in the MD group than in the FB group (−2.9 vs. −0.5 mg/dL; P = 0.0021).
Appetite sensations
A significant increase in the feeling of fullness was noted in the MD group at 52 weeks relative to 26 weeks. However, there were no significant differences in changes in VAS scores regarding hunger, fullness, thoughts of food, urges to eat, and food cravings between the groups comparing scores at week 26 with those at week 52 (data not shown).
Sensitivity analyses
The results of analyses including only those subjects who completed the study on their assigned treatment were not substantially different from the results of the intention-to-treat analysis (Tables 4 and 5). Likewise, although the analyses using last observation carried forward for missing data yielded slightly different results than those presented, there were no substantial interpretive changes (Tables 6 and 7, Supplementary Material).
Table 4. Anthropometric measures, body composition, and blood pressure in the two groups at baseline and follow-up: analysis restricted to participants who completed the study on assigned treatment condition.
| Baseline | Week 26 | Change from baseline | Week 52 | Change from baseline | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||||
| FB (n=45) | MD (n=50) | FB (n=45) | MD (n=50) | FB (n=45) | MD (n=50) | P valuea | FB (n=45) | MD (n=50) | FB (n=45) | MD (n=50) | P valueb | |
| Weight (kg) | 113.1c | 112.0 | 108.7 | 103.5 | −4.4* | −8.5* | 0.0001 | 110.4 | 106.7 | −2.7* | −5.3* | 0.0010 |
| [113.1]d | [112.8] | [108.7] | [104.3] | [−4.4† ] | [−8.5† ] | [0.0094] | [110.5] | [107.5] | [−2.7† ] | [−5.3† ] | [0.0569] | |
| (12.5)e | (17.4) | (13.7) | (17.0) | (7.4) | (8.3) | (13.3) | (17.1) | (6.7) | (7.2) | |||
| BMI (kg/m2) | 41.5 | 40.4 | 39.9 | 37.4 | −1.6* | −3.0* | 0.0003 | 40.6 | 38.6 | −0.9* | −1.8* | 0.0024 |
| [41.4] | [40.6] | [39.8] | [37.6] | [−1.6† ] | [−3.0† ] | [0.0111] | [40.4] | [38.7] | [−0.9† ] | [−1.8† ] | [0.0661] | |
| (3.7) | (3.9) | (4.5) | (4.7) | (2.5) | (2.7) | (4.3) | (4.4) | (2.2) | (2.5) | |||
| Waist (cm) | 111.8 | 107.0 | 107.4 | 100.7 | −4.4* | −6.3* | 0.0073 | 107.6 | 101.5 | −4.2* | −5.4* | 0.0114 |
| [111.9] | [107.2] | [107.4] | [100.9] | [−4.4† ] | [−6.3† ] | [0.0470] | [107.7] | [101.7] | [−4.2† ] | [−5.4† ] | [0.0988] | |
| (10.0) | (10.7) | (10.2) | (11.0) | (5.6) | (5.8) | (10.2) | (11.0) | (4.6) | (5.1) | |||
| Fat mass (kg) | 54.6 | 53.5 | 50.4 | 46.2 | −4.2* | −7.3* | 0.0005 | 52.1 | 48.7 | −2.5* | −4.8* | 0.0021 |
| [54.2] | [53.8] | [50.0] | [46.7] | [−4.2† ] | [−7.4† ] | [0.0173] | [51.6] | [49.0] | [−2.5† ] | [−4.8† ] | [0.0540] | |
| (8.8) | (10.1) | (10.7) | (11.8) | (6.0) | (6.4) | (10.9) | (11.0) | (5.6) | (5.6) | |||
| Fat-free mass (kg) | 58.3 | 58.5 | 58.1 | 57.3 | −0.2 | −1.3* | 0.0203 | 58.2 | 58.0 | −0.1 | −0.5 | 0.1319 |
| [59.0] | [59.1] | [58.8] | [57.8] | [−0.2] | [−1.3† ] | [0.0771] | [58.9] | [58.5] | [−0.1] | [−0.5] | [0.4279] | |
| (9.7) | (11.7) | (9.7) | (10.7) | (2.9) | (3.2) | (9.0) | (11.2) | (2.6) | (2.9) | |||
| Fat-free mass/weight (%) | 51.6 | 52.3 | 53.7 | 55.7 | +2.1* | +3.4* | 0.0039 | 53.0 | 54.5 | +1.4* | +2.2* | 0.0187 |
| [52.1] | [52.3] | [54.2] | [55.7] | [+2.1† ] | [+3.4† ] | [0.0618] | [53.5] | [54.5] | [+1.4† ] | [+2.2† ] | [0.1759] | |
| (5.4) | (5.2) | (6.7) | (6.6) | (3.2) | (3.2) | (6.6) | (6.0) | (3.0) | (2.8) | |||
| SBP (mm Hg) | 124.7 | 123.4 | 121.0 | 119.4 | −3.7 | −4.0* | 0.8032 | 124.4 | 123.2 | −0.2 | −0.2 | 0.9353 |
| [124.7] | [123.6] | [121.0] | [119.6] | [−3.7] | [−4.0† ] | [0.7368] | [124.5] | [123.4] | [−0.2] | [−0.2] | [0.8623] | |
| (13.5) | (13.7) | (11.1) | (13.5) | (11.8) | (13.4) | (12.6) | (14.7) | (9.3) | (10.4) | |||
| DBP (mm Hg) | 74.0 | 73.9 | 73.2 | 72.0 | −0.8 | −1.9* | 0.4548 | 73.8 | 74.7 | −0.2 | +0.8 | 0.3545 |
| [73.9] | [74.1] | [73.1] | [72.2] | [−0.8] | [−1.9† ] | [0.4181] | [73.7] | [74.9] | [−0.2] | [+0.8] | [0.3638] | |
| (7.3) | (7.7) | (7.8) | (8.5) | (5.7) | (7.0) | (7.6) | (8.8) | (5.4) | (5.3) | |||
Abbreviations: BMI, body mass index; DBP, diastolic blood pressure; FB, reduced-energy, food-based diet; MD, Medifast 5 & 1 Plan; SBP, systolic blood pressure.
P <0.05 for within-group difference in change in measure between baseline and week 26 or baseline and week 52 from mixed model analysis of covariance with adjustment for race.
P <0.05 for within-group difference in change in measure between baseline and week 26 or baseline and week 52 from mixed model analysis.
P value for between-group difference in change in measure at week 26 from mixed model analysis of covariance with adjustment for the relevant baseline measure and ethnicity [p-value in parentheses does not adjust for ethnicity as a covariate].
P value for between-group difference in change in measure at week 52 from mixed model analysis of covariance with adjustment for the relevant baseline measure and ethnicity [p-value in parentheses does not adjust for ethnicity as a covariate].
Mean (adjusted for race).
Mean (unadjusted).
Standard deviation.
Table 5. Biochemical measures in the two groups at baseline and follow-up: analysis restricted to participants who completed the study on assigned treatment condition.
| Baseline | Week 26 | Change from baseline | Week 52 | Change from baseline | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||||
| FB (n=45) | MD (n=50) | FB (n=45) | MD (n=50) | FB (n=45) | MD (n=50) | P valuea | FB (n=45) | MD (n=50) | FB (n=45) | MD (n=50) | P valueb | |
| TC (mg/dL) | 188.1c | 179.5 | 187.4 | 170.2 | −0.7 | −9.4* | 0.0045 | 191.3 | 179.5 | +3.2 | 0.0 | 0.1779 |
| [187.8]d | [178.8] | [187.1] | [169.4] | [−0.7] | [−9.4† ] | [0.0036] | [191.0] | [178.7] | [+3.2] | [0.0] | [0.1753] | |
| (42.4)e | (28.4) | (32.1) | (22.4) | (34.1) | (18.4) | (36.6) | (32.0) | (36.6) | (20.1) | |||
| LDL-C (mg/dL) | 118.4 | 107.4 | 116.2 | 97.3 | −2.2 | −10.2* | 0.0010 | 119.7 | 106.0 | +1.3 | −1.4 | 0.1818 |
| [117.8] | [107.2] | [115.7] | [97.0] | [−2.2] | [−10.2† ] | [0.0006] | [119.1] | [105.8] | [+1.3] | [−1.4] | [0.1784] | |
| (34.2) | (26.3) | (26.5) | (24.6) | (18.9) | (17.2) | (30.4) | (31.6) | (27.1) | (16.5) | |||
| HDL-C (mg/dL) | 50.1 | 53.3 | 51.2 | 54.4 | +1.1 | +1.1 | 0.3873 | 50.9 | 55.7 | +0.8 | +2.4 | 0.0686 |
| [49.8] | [53.4] | [50.9] | [54.5] | [+1.1] | [+1.1] | [0.5041] | [50.6] | [55.8] | [+0.8] | [+2.4] | [0.1051] | |
| (11.9) | (14.2) | (13.1) | (10.5) | (10.4) | (8.7) | (11.5) | (12.9) | (8.9) | (9.3) | |||
| TG (mg/dL) | 110.3 | 93.6 | 101.9 | 88.8 | −8.4 | −4.8 | 0.2937 | 104.5 | 88.7 | −5.8 | −5.5 | 0.1198 |
| [112.1] | [91.0] | [103.7] | [86.2] | [−8.4] | [−4.8] | [0.3248] | [106.3] | [85.5] | [−5.8] | [−5.5] | [0.1376] | |
| (50.8) | (46.0) | (44.8) | (39.4) | (42.6) | (33.1) | (48.2) | (27.4) | (44.0) | (37.5) | |||
| Glucose (mg/dL) | 102.1 | 99.3 | 103.4 | 97.3 | +1.3 | −2.0 | 0.0374 | 101.1 | 96.5 | −1.1 | −2.8 | 0.0946 |
| [102.5] | [99.0] | [103.8] | [97.0] | [+1.3] | [−2.0] | [0.0735] | [101.5] | [96.2] | [−1.1] | [−2.8] | [0.1692] | |
| (13.1) | (13.5) | (18.4) | (9.3) | (15.5) | (10.6) | (14.9) | (12.6) | (11.9) | (10.7) | |||
| hs-CRP (mg/L) | 11.6 | 10.3 | 10.4 | 7.9 | −1.3 | −2.4* | 0.0951 | 10.2 | 6.9 | −1.4 | −3.3* | 0.0058 |
| [11.1] | [10.5] | [9.8] | [8.2] | [−1.3] | [−2.4† ] | [0.1936] | [9.6] | [7.2] | [−1.4] | [−3.3† ] | [0.0197] | |
| (10.1) | (8.8) | (10.8) | (6.7) | (5.2) | (4.8) | (8.3) | (6.7) | (5.5) | (5.1) | |||
| ALP (U/L) | 78.4 | 76.3 | 78.6 | 73.4 | +0.3 | −2.8 | 0.1320 | 76.5 | 72.1 | −1.8 | −4.1* | 0.1027 |
| [78.3] | [76.0] | [78.6] | [73.2] | [+0.3] | [−2.8] | [0.1597] | [76.5] | [71.9] | [−1.8] | [−4.1† ] | [0.1352] | |
| (20.9) | (17.8) | (25.0) | (20.4) | (12.1) | (10.2) | (19.8) | (18.0) | (8.7) | (8.2) | |||
| ALT (U/L) | 18.6 | 19.5 | 16.5 | 15.4 | −2.1 | −4.0* | 0.1150 | 17.3 | 15.6 | −1.3 | −3.9* | 0.1456 |
| [18.8] | [19.6] | [16.6] | [15.5] | [−2.1] | [−4.0† ] | [0.1317] | [17.4] | [15.7] | [−1.3] | [−3.9† ] | [0.1627] | |
| (11.3) | (13.1) | (8.5) | (7.4) | (5.7) | (9.2) | (10.5) | (9.9) | (5.7) | (11.0) | |||
| AST (U/L) | 16.6 | 17.7 | 16.9 | 16.1 | +0.3 | −1.6* | 0.0735 | 16.5 | 15.9 | −0.1 | −1.8* | 0.3177 |
| [16.8] | [17.6] | [17.0] | [16.0] | [+0.3] | [−1.6† ] | [0.0399] | [16.6] | [15.8] | [−0.1] | [−1.8† ] | [0.2274] | |
| (6.3) | (7.7) | (4.6) | (3.9) | (4.4) | (6.2) | (5.5) | (6.0) | (3.6) | (7.7) | |||
| LPO (μM) | 8.3 | 7.5 | 5.1 | 4.1 | −3.1* | −3.4* | 0.0109 | 7.3 | 8.6 | −1.0 | +1.1 | 0.2377 |
| [8.2] | [7.5] | [5.1] | [4.1] | [−3.1† ] | [−3.4† ] | [0.0061] | [7.3] | [8.6] | [−1.0] | [+1.1] | [0.2501] | |
| (4.4) | (3.6) | (1.9) | (1.6) | (4.4) | (4.3) | (4.9) | (6.2) | (6.6) | (7.3) | |||
Abbreviations: ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; FB, reduced-energy, food-based diet; HDL-C, high-density lipoprotein cholesterol; hs-CRP, C-reactive protein; LDL-C, low-density lipoprotein cholesterol; LPO, lipid hydroperoxides; MD, Medifast 5 & 1 Plan; TC, total cholesterol; TG, triglycerides.
P <0.05 for within-group difference in change in measure between baseline and week 26 or baseline and week 52 from mixed model analysis of covariance with adjustment for race.
P <0.05 for within-group difference in change in measure between baseline and week 26 or baseline and week 52 from mixed model analysis.
P value for between-group difference in change in measure at week 26 from mixed model analysis of covariance with adjustment for the relevant baseline measure and ethnicity [p-value in parentheses does not adjust for ethnicity as a covariate].
P value for between-group difference in change in measure at week 52 from mixed model analysis of covariance with adjustment for the relevant baseline measure and ethnicity [p-value in parentheses does not adjust for ethnicity as a covariate].
Mean (adjusted for race).
Mean (unadjusted).
Standard deviation.
Discussion
In this RCT targeting obese adults, MD led to significantly greater reductions in body weight, BMI, waist circumference, and fat mass compared to FB at the end of the 26-week weight-loss phase. Furthermore, the MD group maintained significantly greater reductions in weight, BMI, and fat mass following the weight-maintenance phase at 52 weeks. Other notable findings included significantly greater reductions in total cholesterol, LDL cholesterol, and LPO in the MD group at 26 weeks and in hs-CRP at 52 weeks.
Meal-replacement diet plans generally have produced greater weight loss than more traditional diets, at least in the short term. The anthropometric and body composition results following the weight-loss phase in our study generally are in agreement with the results of a previous study comparing the MD to FB diet (10). In that 40-week RCT of 90 obese adults, weight, BMI, waist circumference, and percent body fat showed greater reductions in the MD than in the FB group after the 16-week weight-loss phase. In a study of persons with type 2 diabetes, a portion-controlled meal-replacement diet resulted in greater weight loss at 34 weeks than a reduced-energy, food-based diet (18). Overweight and obese subjects randomly assigned to using liquid meal replacements lost significantly more weight during a 3-month weight-loss period compared with those randomly assigned to an energy-restricted diet (19). A meta- and pooling analysis including six studies utilizing partial meal replacement plans showed a significantly greater weight loss at one year in subjects receiving the partial meal replacements compared to conventional reduced energy diets (20).
Other studies have demonstrated no advantage of meal-replacement diets over traditional low-energy weight-loss diets. In a study in overweight women, weight, waist circumference, and fat mass were reduced to similar degrees after 12 weeks with an energy-restricted diet with or without meal replacements (21). Likewise, there were no significant differences in weight loss at 3 or 6 months in 66 obese subjects randomly assigned to a low-energy/meal-replacement diet or a low-energy only diet (22).
While we continued to see significantly greater reductions in weight, BMI, and fat mass in the MD group after the 26-week weight-maintenance phase (one year after baseline), long-term results of other studies of meal-replacement plans have been mixed. For example, Davis et al. observed no significant differences in changes in weight, BMI, waist circumference, or percent body fat following their 24-week weight-maintenance phase (40 weeks after baseline) (10). However, in another study, although there was no significant difference in weight loss in subjects randomly assigned to a diet plan incorporating liquid meal replacements or an isoenergetic traditional diet after a 12-week intervention period, the meal-replacement group maintained their weight loss, whereas the traditional food group regained most of their weight loss after one year (23). In another study, subjects using meal replacements lost more weight over a 1-year intervention than those attending dietitian-led classes only (24).
Portion-controlled meal replacements may facilitate weight-loss by eliminating the need for dieters to make judgments on appropriate portion sizes (10), which can be challenging in the current environment of expanding portion sizes (11). Meal replacements allow for a more structured meal plan and fewer decisions about food choices on the part of dieters, thus improving compliance and reducing dietary failures (19, 20). In fact, in the current study, more subjects in the MD group than in the FB group completed the study on the assigned treatment, although this difference was not statistically significant. It should be noted, however, that while MD subjects reported that their adherence to the plan was on average slightly better than “fair” at 26 weeks, they reported consuming on average only about half of the total number of recommended meal replacements over the previous 2-week period. It is possible that greater use of the meal replacements would have resulted in greater weight loss.
The effects of meal-replacement diets compared with standard reduced-energy diets on various biomarkers have been equivocal. In contrast to our study, Davis et al. saw no between-group differences in changes in lipids at 16 or 40 weeks (10), nor were there differences in changes in lipids in another such trial following weight-loss or weight-maintenance phases (18), or after a 12-week weight-loss phase in a third trial (21). However, another study showed significantly greater reductions in triglycerides in subjects randomly assigned to a meal-replacement diet compared to those on a standard energy-restricted diet following a 24-month weight-maintenance phase (25). The diminishing reductions in total and LDL cholesterol observed in our study in the weight-maintenance phase compared with the weight-loss phase likely were due to some weight regain (26), noted in both groups during maintenance.
In agreement with our study, no between-group differences in reduction in blood glucose concentrations were noted in subjects on a meal-replacement diet compared with those on a food-based diet (18). However, there were significantly greater reductions in glucose in subjects randomly assigned to a meal-replacement diet compared to a standard energy-restricted diet (25) and in diabetic subjects randomly assigned to liquid meal replacements compared with a standard exchange diet plan (27). In our study, we saw a significant between-group difference in change in hs-CRP at 52 weeks, whereas Davis et al. found this difference only in those with high baseline hs-CRP concentrations (>3.0 mg/dL) (10).
Strengths of this study included the RCT design, the relatively large sample size, the one-year duration of the study, the inclusion of weight-loss and weight-maintenance phases, and the relatively high proportions of subjects completing the study period on their assigned treatment. We attribute the retention of subjects to several factors, including the relationships they developed with the study coordinator, creating a sense of accountability on the part of subjects; the frequency of contacts with subjects (by both telephone and in-person); and the convenient study facilities. MD included actual foods, as opposed to meal replacements such as shakes or bars, and, therefore, may be a more sustainable approach to weight loss. A limitation was not being able to blind participants to the intervention. It is possible that being assigned to an undesirable group (in most cases, the FB according to anecdotal accounts) could have affected compliance with the treatment and ultimately the results. Physical activity, including possible changes in this variable, was not assessed. Finally, subjects in the MD group received their foods at no cost to them, unlike a real-world setting in which individuals would have to purchase meal replacements. Costs associated with meal-replacement diets should be considered when making a recommendation for a weight-loss diet, but were not assessed in this study.
In summary, in obese adults, MD resulted in significantly greater reductions in body weight and fat than did the FB following both a 26-week intervention phase and an additional 26-week weight-maintenance phase. Prepackaged meal-replacement diets appear to have a place in the weight-loss/weight-maintenance arsenal. The balancing of effectiveness, convenience, and ease of use with cost considerations when making recommendations for weight loss is a topic for future research.
Supplementary Material
Acknowledgments
The authors acknowledge Medifast, Inc., Owings Mills, MD, for their support of the study. We acknowledge 9the Metabolism/Human Physiology Core Laboratory of the Nutrition Obesity Research Center, Diabetes Research and Training Center, and Center for Clinical and Translational Science at UAB (grant nos. UL1RR025777, P60DK079626, and P30DK56336, respectively) and those who performed the glucose and hs-CRP assays: Maryellen Williams and Cindy Zeng. We also acknowledge the Bioanalytical Redox Biology Core of the Diabetes Research and Training Center at UAB and those who performed the LPO assay: Gin Chuang, Kelley Johnston, and Douglas R. Moellering, PhD. Finally, we thank Kathryn Kaiser, PhD for her critical review of the manuscript.
Footnotes
Disclosure: Dr. Allison has, anticipates, or has had financial interests with the following: Paul, Weiss, Rifkin, Wharton & Garrison, LLP; Kraft Foods, Inc.; and Nutrition Impact, LLC.
Conflict of Interest: Authors James M. Shikany, Amy S. Thomas, T. Mark Beasley, and Cora E. Lewis have no potential conflicts of interest to report. Dr. Allison has, anticipates, or has had financial interests with the following: Paul, Weiss, Rifkin, Wharton & Garrison, LLP; Kraft Foods, Inc., and Nutrition Impact, LLC, and has received consulting fees from Medifast.
References
- 1.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010. JAMA. 2012;307:491–497. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 2.Ogden CL, Carroll MD, Kit BK, Flegal KM. Centers for Disease Control and Prevention [homepage on the Internet] Washington, DC: Department of Health and Human Services; Prevalence of obesity in the United States, 2009-2010. c2012 - [cited 2012 Jun 5]. NCHS Data Brief No. 82, January 2012. Available from: http://www.cdc.gov/nchs/data/databriefs/db82.pdf. [Google Scholar]
- 3.Fontaine KR, Redden DT, Wang C, Westfall AO, Allison DB. Years of life lost due to obesity. JAMA. 2003;289:187–193. doi: 10.1001/jama.289.2.187. [DOI] [PubMed] [Google Scholar]
- 4.Klein S, Burke LE, Bray GA, Blair S, Allison DB, Pi-Sunyer X, et al. for American Heart Association Council on Nutrition, Physical Activity, and Metabolism. Clinical implications of obesity with specific focus on cardiovascular disease: a statement for professionals from the American Heart Association Council on Nutrition, Physical Activity, and Metabolism: endorsed by the American College of Cardiology Foundation. Circulation. 2004;110:2952–2967. doi: 10.1161/01.CIR.0000145546.97738.1E. [DOI] [PubMed] [Google Scholar]
- 5.Astrup A. Macronutrient balances and obesity: the role of diet and physical activity. Public Health Nutr. 1999;2:341–347. doi: 10.1017/s1368980099000464. [DOI] [PubMed] [Google Scholar]
- 6.Green SM. Obesity: prevalence, causes, health risks and treatment. Br J Nurs. 1997;6:1181–1185. doi: 10.12968/bjon.1997.6.20.1181. [DOI] [PubMed] [Google Scholar]
- 7.Keith SW, Redden DT, Katzmarzyk PT, Boggiano MM, Hanlon EC, Benca RM, et al. Putative contributors to the secular increase in obesity: exploring the roads less traveled. Int J Obes (Lond) 2006;30:1585–1594. doi: 10.1038/sj.ijo.0803326. [DOI] [PubMed] [Google Scholar]
- 8.Bray GA. Lifestyle and pharmacological approaches to weight loss: efficacy and safety. J Clin Endocrinol Metab. 2008;93:S81–S88. doi: 10.1210/jc.2008-1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dansinger ML, Tatsioni A, Wong JB, Chung M, Balk EM. Meta-analysis: the effect of dietary counseling for weight loss. Ann Intern Med. 2007;147:41–50. doi: 10.7326/0003-4819-147-1-200707030-00007. [DOI] [PubMed] [Google Scholar]
- 10.Davis LM, Coleman C, Kiel J, Rampolla J, Hutchisen T, Ford L, et al. Efficacy of a meal replacement diet plan compared to a food-based diet plan after a period of weight loss and weight maintenance: a randomized controlled trial. Nutr J. 2010;9:11. doi: 10.1186/1475-2891-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Young LR, Nestle M. Expanding portion sizes in the US marketplace: implications for nutrition counseling. J Am Diet Assoc. 2003;103:231–234. doi: 10.1053/jada.2003.50027. [DOI] [PubMed] [Google Scholar]
- 12.Derogatis LR, Melisaratos N. The Brief Symptom Inventory: an introductory report. Psychol Med. 1983;13:595–605. [PubMed] [Google Scholar]
- 13.Garner DM, Garfinkel PE. The Eating Attitudes Test: an index of the symptoms of anorexia nervosa. Psychol Med. 1979;9:273–279. doi: 10.1017/s0033291700030762. [DOI] [PubMed] [Google Scholar]
- 14.Flint A, Raben A, Blundell JE, Astrup A. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int J Obesity. 2000;24:38–48. doi: 10.1038/sj.ijo.0801083. [DOI] [PubMed] [Google Scholar]
- 15.Kenward MG, Roger JH. Small sample inference for fixed effects from restricted maximum likelihood. Biometrics. 1997;53:983–997. [PubMed] [Google Scholar]
- 16.Fitzgibbon ML, Tussing-Humphreys LM, Porter JS, Martin IK, Odoms-Young A, Sharp LK. Weight loss and African-American women: a systematic review of the behavioural weight loss intervention literature. Obes Rev. 2012;13:193–213. doi: 10.1111/j.1467-789X.2011.00945.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schulz KF, Altman DG, Moher D for the CONSORT Group. CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Ann Int Med. 2010;152:726–732. doi: 10.7326/0003-4819-152-11-201006010-00232. [DOI] [PubMed] [Google Scholar]
- 18.Cheskin LJ, Mitchell AM, Jhaveri AD, Mitola AH, Davis LM, Lewis RA, et al. Efficacy of meal replacements versus a standard food-based diet for weight loss in type 2 diabetes. Diabetes Educ. 2008;34:118–127. doi: 10.1177/0145721707312463. [DOI] [PubMed] [Google Scholar]
- 19.Ditschuneit HH, Flechtner-Mors M. Value of structured meals for weight management: risk factors and long-term weight maintenance. Obes Res. 2001;9:284S–289S. doi: 10.1038/oby.2001.132. [DOI] [PubMed] [Google Scholar]
- 20.Heymsfield SB, van Mierlo CAJ, Knaap van der HCM, Heo M, Frier HI. Weight management using a meal replacement strategy: meta and pooling analysis from six studies. Int J Obes Relat Metab Disord. 2003;27:537–549. doi: 10.1038/sj.ijo.0802258. [DOI] [PubMed] [Google Scholar]
- 21.Metzner CE, Folberth-Vogele A, Bitterlich N, Lemperle M, Schafer S, Alteheld B, et al. Effect of a conventional energy-restricted modified diet with or without meal replacement on weight loss and cardiometabolic risk profile in overweight women. Nutr Metab. 2011;8:64. doi: 10.1186/1743-7075-8-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Noakes M, Foster PR, Keogh JB, Clifton PM. Meal replacements are as effective as structured weight-loss diets for treating obesity in adults with features of metabolic syndrome. J Nutr. 2004;134:1894–1899. doi: 10.1093/jn/134.8.1894. [DOI] [PubMed] [Google Scholar]
- 23.Rothacker DQ, Staniszewski BA, Ellis PK. Liquid meal replacement vs traditional food: a potential model for women who cannot maintain eating habit change. J Am Diet Assoc. 2001;101:345–347. doi: 10.1016/S0002-8223(01)00089-X. [DOI] [PubMed] [Google Scholar]
- 24.Ashley JM, St Jeor ST, Schrage JP, Perumean-Chaney SE, Gilbertson MC, McCall NL, et al. Weight control in the physician's office. Arch Intern Med. 2001;161:1599–1604. doi: 10.1001/archinte.161.13.1599. [DOI] [PubMed] [Google Scholar]
- 25.Ditschuneit HH, Flechtner-Mors M, Johnson TD, Adler G. Metabolic and weight-loss effects of a long-term dietary intervention in obese patients. Am J Clin Nutr. 1999;69:198–204. doi: 10.1093/ajcn/69.2.198. [DOI] [PubMed] [Google Scholar]
- 26.Leenen R, van der Kooy K, Mayboom S, Seidell JC, Deurenberg P, Weststrate JA. Relative effects of weight loss and dietary fat modification on serum lipid levels in the dietary treatment of obesity. J Lipid Res. 1993;34:2183–2191. [PubMed] [Google Scholar]
- 27.Yip I, Go VLW, DeShields S, Saltsman P, Bellman M, Thames G, et al. Liquid meal replacements and glycemic control in obese type 2 diabetes patients. Obes Res. 2001;9:341S–347S. doi: 10.1038/oby.2001.140. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


