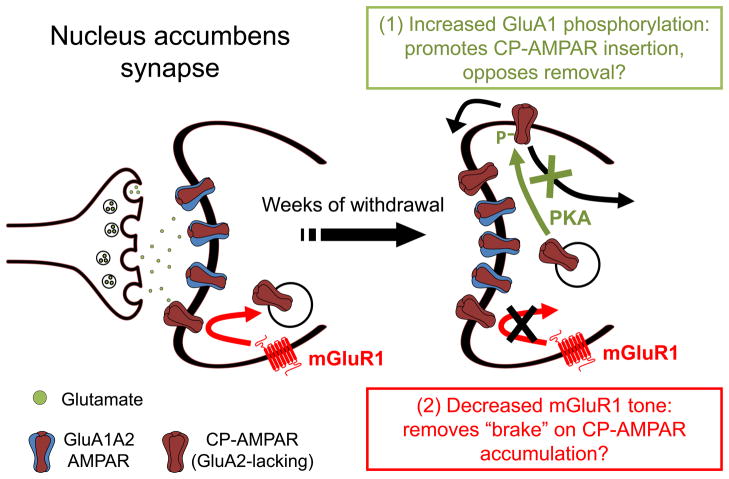
Figure 2. Increased GluA1 phosphorylation and decreased mGluR1 tone may work in concert to enable CP-AMPAR accumulation in the NAc during withdrawal from long access cocaine self-administration.
Synaptic incorporation of GluA1-containing AMPARs in MSNs occurs in two steps: 1) AMPARs from intracellular pools are inserted into extrasynaptic regions of the plasma membrane, and 2) AMPARs are then translocated into the synapse in an NMDAR- and CaMKII-dependent manner. The synapse on the left depicts the situation in drug-naïve rats or in early withdrawal, when GluA2-containing AMPARs (mainly GluA1A2) are responsible for 90–95% of AMPAR transmission. After ~1 month of withdrawal, CP-AMPARs accumulate to the extent that they account for ~30% of AMPAR transmission. We propose that this results from two adaptations that work in concert: 1) Increased phosphorylation of GluA1 on serine 845, and 2) decreased mGluR1 surface expression. Increased phosphorylation of serine 845 is expected to accelerate insertion of GluA1-containing CP-AMPARs from intracellular pools into extrasynaptic portions of the plasma membrane, increasing the size of extrasynaptic AMPAR pools that supply the synapse and thereby increasing the likelihood that CP-AMPARs will enter the synapse. Phosphorylation of serine 845 may also stabilize CP-AMPARs by preventing their internalization and degradation. See Section 4 for more discussion and citations. As in other brain regions, mGluR1 negatively regulates synaptic levels of CP-AMPARs in the NAc. A withdrawal-dependent decrease in mGluR1 surface expression in the NAc, by removing this “braking effect”, may enable CP-AMPARs to accumulate. See Section 3 for more discussion and citations. PKA, protein kinase A.