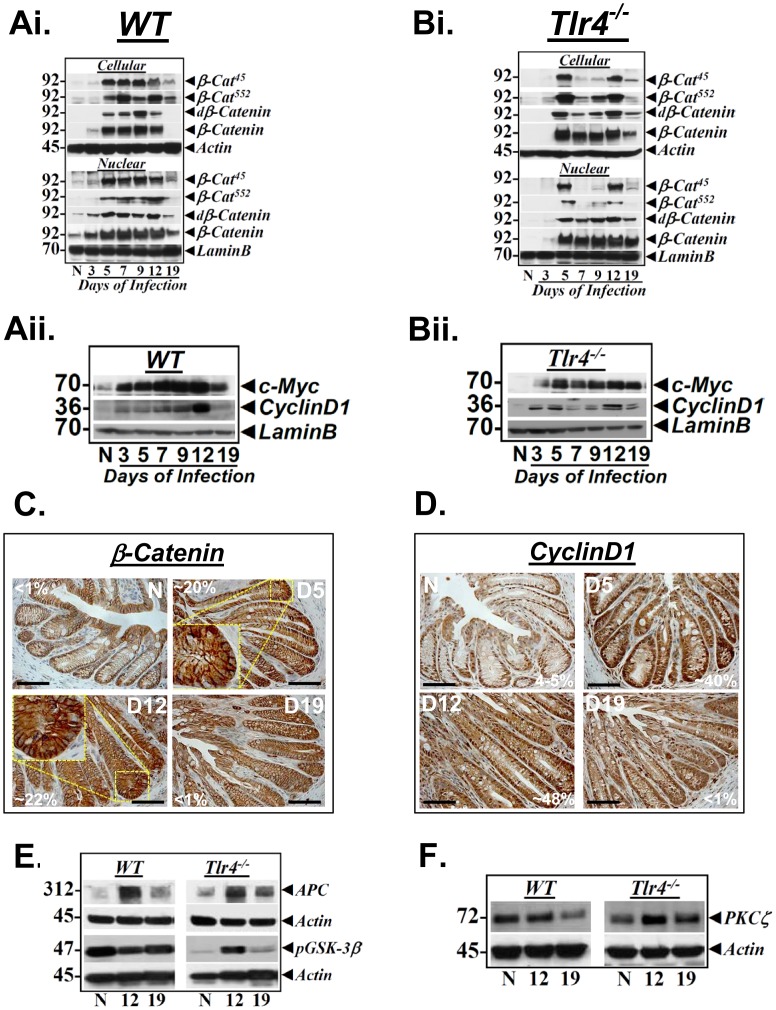
Figure 3. Effect of CR infection on Wnt/β-catenin signaling in vivo.
Relative levels of phosphorylated (β-Cat45/β-Cat552), de-phosphorylated (dβ-Catenin) and total β-catenin in the cellular and nuclear extracts prepared form the uninfected normal (N) and days 3–19 post infected wild type (WT) C57Bl/6 (Ai) and Tlr4−/− (Bi) mice were determined by Western blotting with moiety-specific antibodies. Actin or LaminB were loading controls. Downstream targets of Wnt/β-catenin signaling are upregulated in response to CR infection. Relative levels of cyclinD1 and c-Myc in the nuclear extracts prepared from the uninfected normal (N) and days 3–19 post infected wild type (WT) C57Bl/6 (Aii) and Tlr4−/− (Bii) mice were determined by Western blotting. LaminB was used as loading control. C and D. Immuno-staining for β-catenin and cyclinD1 in Tlr4−/− mice. Paraffin embedded sections prepared from the distal colons of uninfected normal (N) or 5, 12 and 19-days post-CR infected Tlr4−/− mice were stained for β-catenin (C) or cyclinD1 (D) respectively. Inset shows diffused nuclear/peri-nuclear staining. Percentages represent percent nuclear positivity for indicated proteins. Scale bar: 50 µm; n = 2 independent experiments. E and F. Increases in β-catenin are not due to functional inactivation of APC protein. Western blots showing relative levels of APC (E), GSK-3β phosphorylated at Ser-9 (E) and PKCζ (F) in the cellular crypt extracts prepared form the uninfected normal (N) and days 12 and 19 post infected wild type (WT) C57Bl/6 and Tlr4−/− mice, respectively (n = 3 independent experiments).