Abstract
Streptomyces phage φBT1 integrates its genome into the attB site of the host chromosome with the attP site to generate attL and attR. The φBT1 integrase belongs to the large serine recombinase subfamily which directly binds to target sites to initiate double strand breakage and exchange. A recombination directionality factor (RDF) is commonly required for switching integration to excision. Here we report the characterization of the RDF protein for φBT1 recombination. The RDF, is a phage-encoded gp3 gene product (28 KDa), which allows efficient active excision between attL and attR, and inhibits integration between attB and attP; Gp3 can also catalyze topological relaxation with the integrase of supercoiled plasmids containing a single excision site. Further study showed that Gp3 could form a dimer and interact with the integrase whether it bound to the substrate or not. The synapse formation of attL or attR alone with integrase and Gp3 showed that synapsis did not discriminate between the two sites, indicating that complementarity of central dinucleotides is the sole determinant of outcome in correct excision synapses. Furthermore, both in vitro and in vivo evidence support that the RDFs of φBT1 and φC31 were fully exchangeable, despite the low amino acid sequence identity of the two integrases.
Introduction
Bacteriophages typically insert their genomes into host chromosomes via integrase-mediated site-specific integration between two sites of attB and attP from bacteria and phage, respectively, and form attL and attR sites to establish a lysogenic state. Under inducing conditions, the phage genome is eliminated via site-specific excision between attL and attR to convert into the lytic life cycle [1], [2]. The phage encoded integrase protein is required for both integration and excision [3], [4], however, this process is highly unidirectional and controlled by a recombination directionality factor (RDF or Xis) [1], [3], [5], [6], [7].
Phage-encoded site-specific integrases have been classified into two major groups: tyrosine and serine recombinases, which contain tyrosine or serine to attack DNA substrates in the active sites of the proteins [5]. The best studied member of the tyrosine recombinase group is that from E.coli phage λ [8], which has been extensively investigated both biochemically and structurally for decades [8], [9], [10], [11], [12], [13]. The recombination sites for λ integrase are quite different, as the attB site is short (25 bp) and simple, while the attP site is relatively large (240 bp) and contains multiple binding sites for integrase, Xis, IHF (integration host factor) and Fis (factor for inversion stimulation) [14], [15]. Xis is the master regulator of λ recombination, and three Xis-binding sites have been found in attP [16]. DNA bending catalyzed by Xis promotes the formation of an excisive intasome, but inhibits the formation of the integrative intasome [13].
In contrast, the mechanism of serine recombinases is less well understood, and the γδ resolvase is a member of the better known serine recombinases [5], [17], [18]. A number of phage-encoded integrases which were classified into the large serine recombinase subgroup [19], typically contain a large C-terminus which may be involved in DNA binding and directionality control [5], [20]. The best studied of these integrases in vitro are those of the Streptomyces phages φC31 [21], φBT1 [22], [23] as well as TG1 [24], and the mycobacterial phages Bxb1 [25] and φRv1 [4]. In each of these cases, the recombination sites of attB and attP are simple and short (less than 50 bp), and contain central dinucleotides of crossover sites which may control the polarity of the recombination [26]. To date, RDFs of phage TP901-1 [27], Bxb1 [3], φRv1 [4] as well as φC31 [1] have been identified and actively allow excision between attL and attR, and inhibit integration between attB and attP. A DNA binding assay of φC31 and Bxb1 Xis proteins strongly supported the view that Xis interacts with the att-bound integrases to change the conformation of the complexes that favor proceeding to excisive recombination [1], [3], [5]. Furthermore, two new reports on large serine recombinases, one of a single-molecule analysis of Bxb1 recombination revealed the molecular bearing mechanism of DNA strand exchange [28], and the second on φC31 integrase using hybrid “phes” recombination sites proposed a gated rotation mechanism [29]; both strongly support the “subunit rotation” model for exchanging DNA strands [30].
Streptomyces phage φBT1 is a temperate phage related to φC31 that integrates its genome into SCO4848 coding a putative integral membrane protein of Streptomyces coelicolor [31]. We previously established a highly efficient site-specific in vitro recombination system based on purified φBT1 integrase, and determined the minimal sites of attB and attP [23]. The biochemical mechanisms underlying synapsis, strand cleavage and rejoining were further studied, and a model in which two alternative pathways can lead to synaptic complex formation of integration was proposed [22]. Furthermore, φBT1 integrase-based methods have also been widely used in various Streptomyces strains [31], [32], [33], [34], mammalian cells [35] and in vitro DNA assembly [36]. However, the directionality control factor of the φBT1 recombination system has not been identified to date. Here, we aim to characterize the RDF that regulates the directionality of recombination catalyzed by φBT1 integrase. The RDF protein of φBT1, which is encoded by phage gp3 gene, was sufficient to activate in vitro excisive recombination and inhibit integrative recombination.
Materials and Methods
Strains, bacteriophages and plasmids
E.coli and the Streptomyces strains used in this study are described in Table S1 in File S1. Construction of phagemid φXD101 was as follows: A 3037 bp PCR product containing gp15 to gp22 of phage φC31 using primers 5′-ACTCTAGAGCCGAAGGCGCCACGCAA-3′ and 5′-GCGGATCCGTCGCTGGGTGGACGTAC-3′ was amplified and digested with XbaI and BamHI, and inserted into the XbaI-BamHI-cleaved plasmid, pSET152, to generate a gene-targeting vector pDXM101. Plasmid pDXM101 was then introduced into Streptomyces coelicolor strain J1929 containing a φC31 lysogen by conjugation from E.coli strain ET12567/pUZ8002. After selected on Apramycin agar, the positive colonies, mixed with both homologous recombinants targeting φC31 prophage and integrative exconjugants, were pooled. Spores of those colonies were used for the burst of phages, plated onto soft agar with indicator strain (spores of wild type Streptomyces coelicolor strain J1929) to yield plaques. The apramycin resistant phage was then obtained by transformation of the isolated phage DNA into E.coli strain DH10B and selected with antibiotics. That resulting phagemid, designated φXD101, can be maintained in E.coli as a plasmid, and conjugated into Streptomyces as an active phage. The phage genes gp23 to gp28 were deleted during the process of homologous recombination followed by phage packaging to adapt the phage genome size. Construction of plasmid-phage φXD101(X02) and φXD101(X03) by the PCR-targeting method [37] was as described in the Results and Discussion. Details of the construction of other plasmids and primers used in this study are described in Table S1 and Table S2 in File S1.
Protein expression, purification and crosslinking
Expression and purification of φBT1 and φC31 integrase were as described in our previous work [22], [23]. For φBT1 excisionase (Gp3), the gp3-φBT1 gene (accession number AJ550940.2) was chemically synthesized and cloned into expression vector pET-28b (+) to generate pET-28-Bxis, that now carries a poly-His tag fused at the N-terminus of the gp3-φBT1 gene. In addition, the φC31 excisionase, the gp3-φC31 gene, was PCR amplified from phage φC31 and cloned into pET-28b (+) (see Table S1 in File S1). The procedures for protein expression, purification [23] and crosslinking [38] were performed as described previously.
In vitro recombination and relaxation assay
Standard recombination was carried out in a reaction mixture (10 µl) containing 10 mM Tris-HCl at pH 8.0, 100 mM KCl, 5% glycerol and integrase (270 nM) [23] with or without excisionase (0.25 µl),except where otherwise indicated. The final concentration of monovalent cations (K+ and Na+) was 150 mM. Reactions using linear DNA substrates were incubated at 30°C for 30 min (or as indicated) and were terminated by heat inactivation at 75°C for 10 min or treated with proteinase K at 55°C for 30 min; products were separated by electrophoresis on agarose gels (0.8%). Reactions using supercoiled plasmids for the quantification of recombination efficiency were treated with proteinase K at 55°C for 30 min followed by transformation into E.coli strain DH10B.
Topological relaxation assays were performed with supercoiled plasmids containing attB (plasmid pZLB00), attP (plasmid pZLP00), attL (plasmid pZL5816), and attR (plasmid pZL5817) (see Table S1 in File S1). The DNA substrates were incubated with φBT1 integrase and (or) excisionase for 1 hour. Reactions were carried out similar to the in vitro recombination assays; however, the reactions were terminated by heat inactivation at 75°C for 10 min and separated by electrophoresis on agarose gels (0.8%) in TAE buffer, and visualized by post-staining with ethidium bromide (EtBr). The bands between the supercoiled DNA and relaxed circles displayed a “ladder” of closed circular DNA species, which were likely topoisomers with a decreasing number of knots.
Electrophoretic mobility shift assay (EMSA)
FAM-labeling of DNA fragments was performed by PCR using the primer ZL93 5′-labeled with 5-FAM. attB212 was amplified from pZLB00 using primers ZL95/ZL80 and then labeled using primers ZL93/ZL80; attP247 was amplified from pZLP00 using primers ZL94/ZL82, and then labeled using primers ZL93/ZL82; attL306 was amplified from pZL5819 using primers ZL95/ZL88 and then labeled with primers ZL93/ZL88; attR153 was amplified from pZL5819 using primers ZL94/ZL80 and then labeled with ZL93/ZL80. Approximately 0.1 pmol (10 ng) of FAM-labeled attB212, attP247, attL306 or attR153 DNA were incubated with the indicated amounts of integrase in a binding mixture (10 µl) containing 20 mM Tris-HCl at pH 8.0, 100 mM KCl, 1 mM DTT, 5% glycerol and 500 ng of sonicated salmon sperm DNA; reactions were incubated at 30°C for 30 min and separated on 5% non-denaturingpoly-acrylamide gels in 1×TBE buffer at 4–10°C. DNA bands were visualized by fluorescence imaging using an FLA-9000 Starion Image Scanner (Fuji Film).
Plaque assay
S.coelicolor spores of lysogen J1929 harbouring φXD101, φXD101(X02) and φXD101(X03) (1×106 cfu) were incubated in 30 ml 2×YT medium at 30°C with shaking for 16 hrs. The cultures were filtered using a 0.45 µm filter membrane to obtain phage suspension, and 10 µl of the suspensions were pipetted onto Difco nutrient broth (DNB) agar with MgSO4 (10 mM) and Ca(NO3)2 (8 mM). The soft DNB top layers (containing spores of indicator strain J1929, 1×108 cfu) were then added to each plate, and the plates were incubated overnight at 30°C to generate plaques.
Results and Discussion
Gp3 is the RDF in phage φBT1 recombination
Streptomyces phage φBT1 integrase-mediated site-specific recombination is highly efficient both in vivo [31], [35] and in vitro [22], [23], and has become a very useful tool in a variety of applications [32], [36]. However, the recombination directionality factor (RDF) of φBT1 has not yet been identified, and limits the further development of this system. The genome organization of phage φBT1 is highly similar to that of φC31, and the major gene products are closely related [31]. Previous studies have shown that the early phage protein, Gp3, from φC31 is an RDF of recombination which activates excision and inhibits integration [1], [39]. Amino acid sequence alignment of the Gp3 from φBT1 and φC31 showed that the two proteins shared 85% identity (see Figure S1 in File S1) [32]; it is likely that the Gp3 of φBT1 has the same function as Gp3-φC31 as an RDF. Thus, the gp3-φBT1 gene was chemically synthesized and cloned into the expression vector. Details of protein expression and purification are described in Materials and Methods. As shown in Figure S2 in File S1, Gp3-φBT1 was isolated with a purity over 95%, and was analyzed by both SDS-PAGE and gel filtration (data not shown).
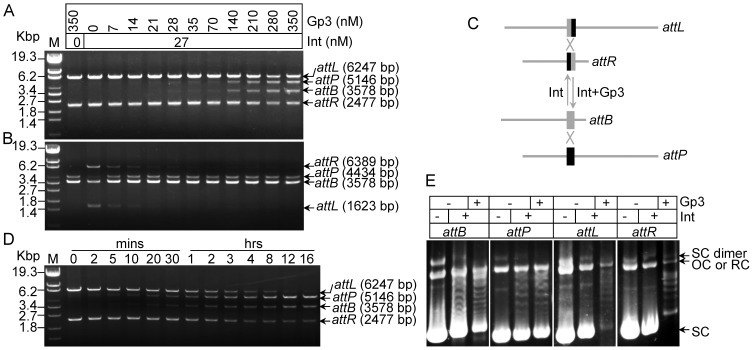
Then in vitro excision and integration assays were performed using linearized substrates. Considering the apparent binding affinities (Kd) of integrase to the attB, attP, attL and attR sites observed at 60 nM in our previous study [22], approximately half of the concentration of integrase (27 nM) was used for primary assays (Figure 1A and 1B). The excision products (attB and attP) could be detected when an equal amount of Gp3 (28 nM) was added, and the productivities increased gradually with increasing amounts of Gp3. An obvious excision band was observed at 140 nM of Gp3; and inhibition of integration was detected when a little Gp3 (7 nM, 1/4 of Int) was added to the reaction (Figure 1B). This observation are consistent with the results observed in mycobacteriophage φRv1 excision, where 50% product formation was reduced by adding as little as 24 nM of RDF(concentration of integrase was 400 nM) [4]. However, it is different from that found in the φC31 recombination, that is, equal to or greater than 1∶2 Gp3 to Int was sufficient to inhibit integration [1]. Our data indicated that Gp3 monomer might interact with Int tetramer in the synaptic complex to inhibit integration.
Figure 1. Gp3 with integrase catalyze excision in phage φBT1 recombination.
(A and B) In vitro excision and integration recombination using linearized DNA in the presence of Gp3 and integrase. For the substrates of excision, plasmid pZL5813 was digested with KpnI to generate attL (6247 bp) and attR (2477 bp); the product sizes were predicted as 3578 bp for attB and 5146 bp for attP. For integration, pZL5812 was digested with HindIII to generate attB (3578 bp), and pZL5811 was digested with EcoRI to generate attP (4434 bp); the product sizes were predicted as 1623 bp for attL and 6389 bp for attR. Substrates were incubated with or without 27 nM integrase and varying concentrations (nM) of Gp3 for two hours. (C) Schematic diagrams of substrates used and the expected products in the excision and integration reactions shown in (A). (D) Time course of in vitro excision, and the substrates used were as shown in (A). The concentrations of proteins were 270 nM for integrase and 350 nM for Gp3. The reaction times are indicated. (E) DNA topological relaxation assays of attB (plasmid pZLB00), attP (plasmid pZLP00), attL (plasmid pZL5816), and attR (plasmid pZL5817) were performed with or without integrase or Gp3. The bands between the supercoiled and relaxed circles formed a ladder of closed circular DNA species that are probably topoisomers with a declining degree of superhelicity. The positions of supercoiled substrate DNA (SC), relaxed circles (RC), open circles (OC) and dimeric supercoiled DNAs (SC dimer) are indicated.
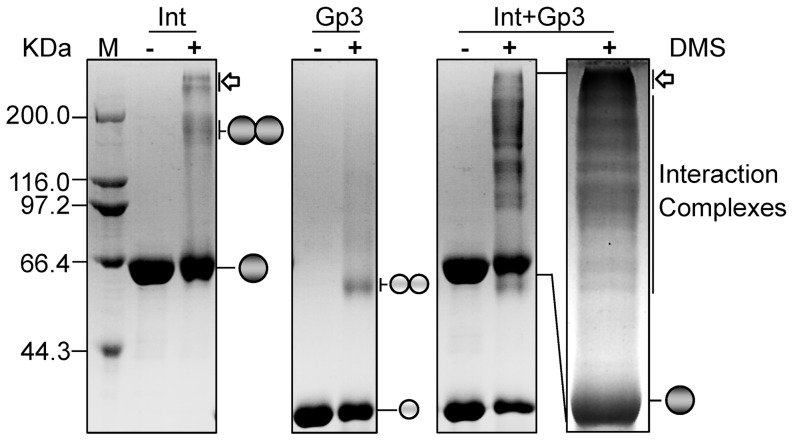
To determine the polymeric form of the Int and Gp3, crosslinking experiments were performed using purified proteins. As shown in Figure 2, both the Int and Gp3 formed dimers in solution; and when the two proteins were mixed together, a ladder of higher order oligomers was observed, indicating that a series of intermediate oligomers were formed. These results confirmed the protein-protein interactions of Int and Gp3 in solution; and indicated that these interactions could occur with different numbers of Int and Gp3 molecules, before obtaining the appropriate stoichiometry.
Figure 2. Crosslinking of φBT1 integrase and Gp3.
Enzymes were incubated in the absence or presence of dimethyl suberimidate (DMS) and analyzed by SDS-PAGE (see Materials and Methods). The positions of integrase (Int) monomer and dimer, Gp3 monomer and dimer, as well as possible interaction complexes are illustrated. The bands indicated by hollow arrows could be higher order oligomers. M, protein molecular weight markers.
To investigate the kinetics of excision, the time course of in vitro excision was studied. The excision recombination showed relatively slower kinetics than that of integration [23], where 50% of products were observed in 0.5 hours; however, excision took 2 hours (Figure 1D). Furthermore, our previous study showed that integrase could catalyze the topological relaxation of supercoiled plasmids containing single integration sites (attB or attP) in a partner DNA-independent manner, but not for the excision sites (attL or attR) [22]. Therefore, topological relaxation assays of plasmids containing attB, attP, attL or attR were performed with or without Int or Gp3(Figure 1E). Topological relaxation catalyzed by φBT1 integrase using supercoiled attL or attR plasmids was observed following the addition of Gp3; and no inhibition of relaxation on supercoiled attB or attP plasmids was observed (Figure 1E), indicating that the presence of Gp3 had no effect on the DNA binding and cleavage activity of integrase on the single integration sites.
DNA binding properties of Gp3
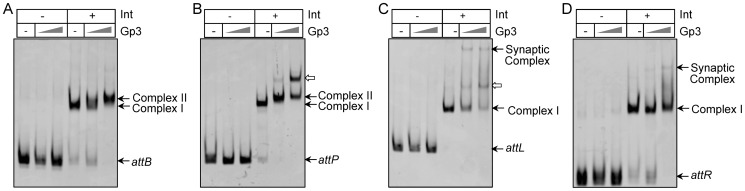
To test the ability of Gp3 to bind to substrates attB, attP, attL and attR, electrophoretic mobility shift assay (EMSA) was performed using FAM-labeled attB212, attP247, attL306 and attR153. As shown in Figure 3, no DNA binding activity was detected when Gp3 was the only protein in the reactions, including at a high concentration of 1750 nM. Thus, similar to the RDFs of phage Bxb1 and φC31 [1], [3], the Gp3 of φBT1 had no DNA binding ability to the target substrates.
Figure 3. Gp3 acts as the RDF through interaction with integrase.
Investigation of DNA binding properties of Gp3 with or without integrase (Int) using FAM-labeled attB212 (A), attP247 (B), attL306 (C) and attR153(D). The Int concentration in the reactions was 135 nM, and concentrations of Gp3 were 175 nM and 1750 nM. The positions of free DNA, complex I, complex II and the synaptic complex are indicated. The bands indicated by hollow arrows in (B) and (C) could result from multiple Gp3 monomers interacting with the Int dimer at higher concentrations.
We further explored the properties of integrase bound to DNA in the presence of Gp3, since protein-protein interactions were observed in Figure 2. As shown in Figure 3A and 3B, integrase could bind to attB or attP to form “Complex I”; and after Gp3 was added, a slower migrating complex (complex II) was detected. This observation suggested that Gp3 might interact with integrase and form a stable complex to inhibit integration between attB and attP [1], [3], [5]. Nevertheless, the slower migrating complex (complex II) was not observed when substrates attL or attR were tested(Figure 3C and 3D); this result was consistent with the DNA binding properties in Bxb1 excision, where the RDF of Bxb1 might bind weakly to the attL/R-Int complexes and fail to be detected during gel electrophoresis [3]. However, slower migrating complexes were detected when integrase and Gp3 of φC31 were incubated with attL or attR [1]. It is interesting that although the RDF for φBT1 shared much higher identity of the amino acids sequences with φC31 than that of mycobacteriophage RDF for Bxb1, the effects on the conformations of attL/R-Int complexes may be diverse, or scarcely show any association with similarity of the amino acids sequences.
Complementarity of central dinucleotides is the sole determinant of recombination outcomes in the correct excision synapses
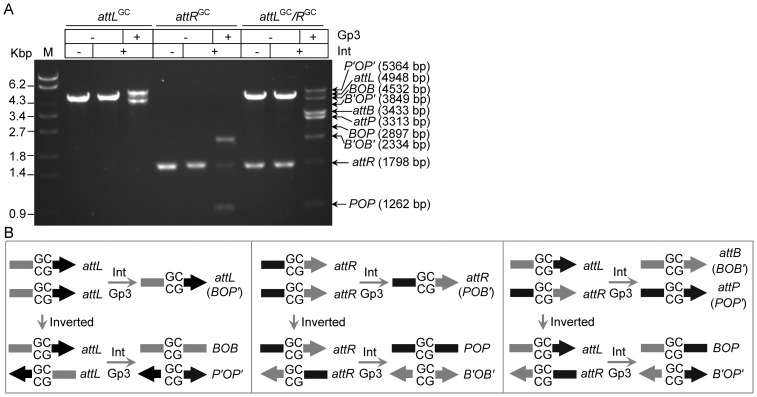
Interestingly, a very slow-migrating band was formed, as shown in Figure 3C and 3D, when single substrate attL or attR alone was incubated with Int and Gp3 in the reaction. However, this was not observed in the excision of φC31 [1]. This might be a consequence of attL or attR synapses with itself. It raised a question regarding attL or attR that each could be involved in recombination with itself. Thus, excision reactions were performed using substrates containing palindromic central dinucleotides (attLGC and attRGC). As shown in Figure 4, both attLGC and attRGC could participate in recombination with itself, and the unnatural products were generated by anti-parallel alignment of the substrates, i.e., BOB and P′OP′ for attL, POP and B′OB′ for attR (Figure 4B). Furthermore, there is the possibility of generating recombination products by parallel alignment of substrates; however, as shown in Figure 5, the relative recombination efficiencies of attL/attL and attR/attR were only 4% and 3%, respectively. Thus, these data supported the view that the slow-migrating band shown in Figure 3C and 3D was accumulation of synaptic complexes caused by anti-parallel alignment of attL or attR with itself, which contained 5′-GT central dinucleotides and further religation was suspended in the synapsis.
Figure 4. Substrates with palindromic central dinucleotides participate in attL/attL and attR/attR excision.
(A) Excision reactions were performed using attL and attR with palindromic central dinucleotides. Plasmid pZLLR03 was digested with EcoRI and AgeI to generate linearized attLGC (4948 bp) and attRGC (1798 bp). The product sizes were predicted as 4532 bp for BOB and 5364 bp for P′O P′, 1262 bp for POP and 2334 bp for B′OB′, 3433 bp for BOB′(attB) and 3313 bp for POP′(attP), 2897 bp for BOP and 3849 bp for B′OP′. The positions of the substrates and series of products are indicated. The concentrations of proteins were 270 nM for integrase and 350 nM for Gp3. The reactions were terminated by proteinase K after incubation at 30°C for eight hours. (B) Schematic diagrams of the excision reaction shown in (A). Both attL and attR may form parallel and anti-parallel alignment, however, only correct alignments of substrates could participate in correct excision synapses, followed by excision.
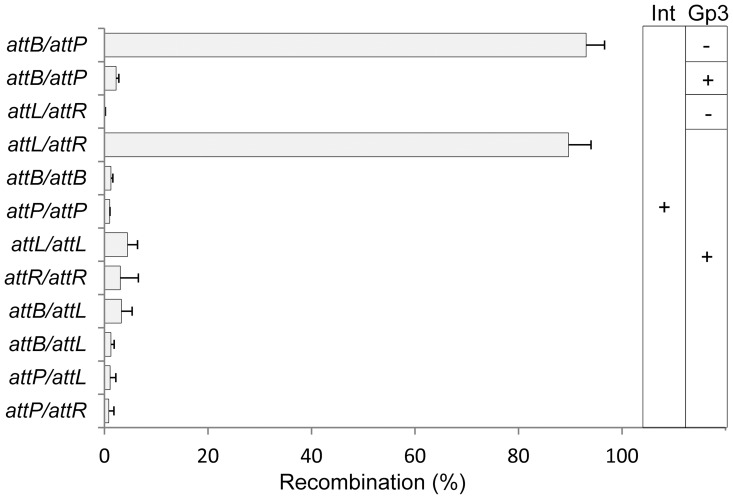
Figure 5. Quantification of in vitro excision efficiencies between two sites of attB, attP, attL or attR.
In vivo detection of in vitro recombination products by blue-white screen; the four recombination targets were inserted into lacZα such that LacZ activity was abolished by recombination. The four target plasmids were pZLB00 (atttB), pZP00 (atttP), pZLL00 (atttL) and pZLR00 (atttR); the plasmids containing partner DNA were pZL5812 (attB), pZLP00 (attP), pZL5816 (attL) and pZL5817 (attR). After in vitro recombination, the plasmids were transformed into E.coli DH10B and plated on IPTG/X-gal medium; the white clones were produced by recombination. The substrate ratio of each combination was 5∶1 (partner to target). The data shown are average values of three reactions. The substrates used in each reaction and the relative recombination efficiencies (%) are indicated.
To gain further insights into the substrate alignments and formation of correct excision synapses in the recombination, attLGC and attRGC were incubated with integrase and Gp3 in one reaction. As shown in Figure 4, the natural excision products of attB and attP were detected; however, the unnatural products were formed with equal efficiency. Thus, the products for three forms of anti-parallel alignment of the substrates, i.e., attL/attL, attR/attR and attL/attR, were observed; and the product (BOP and B′OP′) for parallel alignment of attL and attR was detected with very low yield. Considering the low selectivity to the arm sequences of attB and attP in our previous study [22], it is believable that synapsis does not discriminate between attL and attR as represented in Bxb1 excision [40]. Thus, complementarity of central dinucleotides is the sole determinant in recombination outcomes once the correct excision synapses are formed. This property is consistent with that observed in excision recombination of Bxb1, φRv1 and φC31 [1], [20], [40].
Substrate selection in the presence of integrase and Gp3
To address the substrate specificity of excision recombination, we used a quantitative in vitro excision assay. Reporter plasmid substrates were constructed in which the target sites were inserted in-frame into lacZα of plasmid pBC-SK(−). Following in vitro recombination with partner plasmid, the products were transformed into E.coli and plated on indicator media: white colonies indicated that recombination had occurred. As demonstrated in Figure 5, the frequency of white colonies was more than 90% when integration occurred between attB and attP; however, the efficiency was reduced to 2% in the presence of Gp3. Typical excision catalyzed by integrase and Gp3 between attL and attR gave 89% white colonies. No substantial amounts of white colonies were observed between other combinations of target and partner plasmids, although those between attL/attL (4%), attR/attR (3%) and attB/attL (3%) showed relatively higher efficiencies. However, it seems that excision of φBT1 showed more stringent substrate selectivity than that of φC31, where white colonies of 36% for attL/attL and 18% for attR/attR were observed [1].
Cross-functional excision of integrase with Gp3 from φBT1 and φC31 respectively
Since the Gp3 proteins from phage φBT1 and φC31 shared 85% identity of amino acid sequences (see Figure S1 in File S1), it is reasonable to believe that the two proteins were exchangeable during in vitro recombination. The gp3-φC31 gene was then PCR amplified from phage φC31 and cloned into the expression vector, and the Gp3-φC31 was purified to near homogeneity (see Figure S2 in File S1).
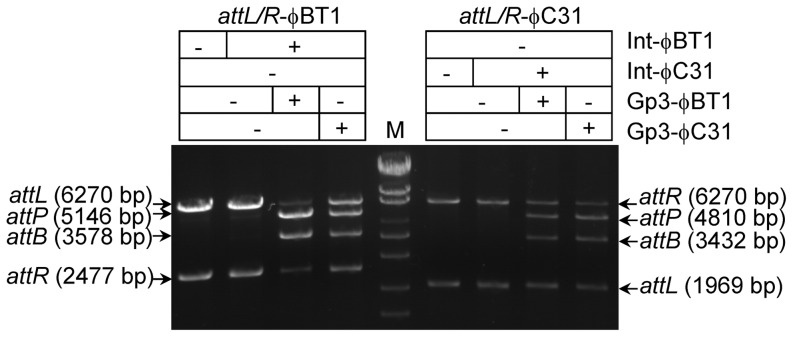
In vitro excision assays were performed both using linearized substrates of φBT1 and φC31. As shown in Figure 6, both Gp3-φBT1 and Gp3-φC31 could catalyze efficient excision between attL/attR-φBT1 or attL/attR-φC31 with their corresponding integrases. It is interesting that the φBT1 and φC31 integrases only showed 26% identity of amino acid sequences; their RDFs, however, shared very high identity (85%) and were proved replaceable through in vitro recombination assays (Figure 6). This suggested that the protein structures to interact with RDFs of φBT1 and φC31 integrases might be highly similar despite the low identity of amino acid sequences. Furthermore, the Streptomyces phage TG1 [24], [41] protein Gp25 (26.4 KD) shared 62% identity with Gp3-φBT1, and 60% of that with Gp3-φC31(see Figure S1 in File S1); suggesting the similar function of Gp25 in TG1 recombination. Furthermore, among four previous experimentally identified RDFs for large serine recombinases, Xis of the mycobacteriophage φRv1 (GpRv1584c, 8KD) [4] and lactococcal phage TP901-1 (Orf7, 7.5 KD) [27] are relatively small; Gp3 (27 KD) of Streptomyces phage φC31 [1] and Gp47 (28 KD) of mycobacteriophage Bxb1 [3] are larger. And they shared no identity of amino acid sequences, which is consistent with the diversity of RDFs [7].
Figure 6. In vitro excision using Gp3 from φBT1 and φC31.
Excision reactions were performed using attL and attR of φBT1 or φC31. attL and attR of φBT1 were obtained as described in Figure 1. For substrates of φC31, plasmid pZL5822 was digested with EcoRI to generate attL-φC31(1969 bp) and attR-φC31 (6270 bp); the product sizes were predicted as 3432 bp for attB φC31 and 4810 bp for attP-φC31. Pairs of excision substrates were incubated with their own integrase in the presence or absence of Gp3 from φBT1 or φC31. The concentrations of proteins used were 270 nM for integrase and 350 nM for Gp3. The reactions were terminated by proteinase K after incubation at 30°C for eight hours.
Gp3 of φBT1 could serve as the RDF in φC31 excision out of the host genome
To confirm the cross-functional excision property of integrase with Gp3 from φBT1 and φC31 in vivo, plaque assays of mutated phage φC31 were performed. Neither integration nor excision is typically essential for lytic propagation following phage infection; however, excision is required for productive lytic growth from a prophage. To test this, we constructed lysogen harboring φC31 with gp3 deletion, wondering if the phage release will decrease and that mutation could be complemented by gp3-φBT1.
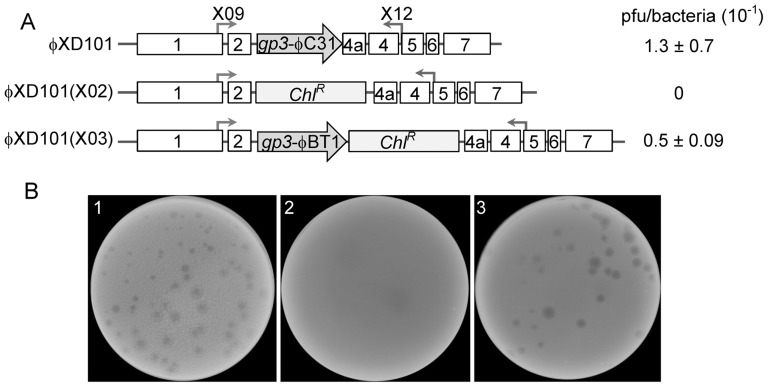
A φC31 derivative phagemid φXD101 was generated, which was maintained in E.coli as a plasmid, and conjugated into Streptomyces as an active phage. Details of the construction of φXD101 are described in Materials and Methods. As illustrated in Figure 7A, the gp3-φC31 gene of φXD101 was replaced by Chloramphenicol resistant gene (ChlR) to generate φXD101(X02) using the PCR-targeting system [37]; and further replaced by gp3-φBT1 and ChlR to obtain φXD101(X03). PCR analysis using primers X09/X12 confirmed the successful construction of the three plasmids (see Figure S3A in File S1). The plasmids were then introduced into Streptomyces coelicolor indicator strain J1929 by conjugation, and Figure S3B in File S1 shows the PCR verification of the positive exconjugants of wild-type S.co J1929 and harboring φXD101, φXD101(X02) or φXD101(X03).
Figure 7. Plaque assay of mutated phage φC31.
(A) Schematic representation of gp3 and surrounding phage genes. Plasmid-phages φXD101(X02) and φXD101(X03) were derivatives of φXD101 by PCR-targeting technology. gp3 of φXD101 was replaced by Chloramphenicol resistant gene (ChlR) in φXD101(X02), and further replaced by gp3-φBT1 and ChlR in φXD101(X03). The construction of φXD101(X02) and φXD101(X03) are described in Materials and Methods. Primers X09 and X12 were used for identification of the derivatives, and the results are shown in Figure S3 in File S1. (B) Plaque assays of phage φXD101, φXD101(X02) and φXD101(X03). The three plasmids were transformed into Streptomyces coelicolor strain J1929 by conjugation from E.coli strain ET12567/pUZ8002 and selected on Apramycin agar; the positive clones were isolated to burst phages. The phage suspension was then plated onto soft agar with spores of indicator strain to yield plaques. Plates 1, 2 and 3 were prepared using suspensions from J1929 harbouring φXD101, φXD101(X02) and φXD101(X03), respectively.
As shown in Figure 7B, plaques were clearly formed when using phage suspension from strains harboring gp3-φC31 as well as gp3-φBT1; however, no plaques of the gp3-φC31 deletion construct were detected. The quantitative analysis of pfu/bacteria is shown in Figure 7A. The phenomenon that no plaques detected on the second plate was over expected. It seems like the RDF Gp3-φC31 or Gp3-φBT1, is required both for prophage excision and phage DNA replication, as described in Bxb1 RDF Gp47 [42]. This observation supported that Gp3-φBT1 shared identical function with Gp3-φC31 in vivo, could serve as the RDF in φC31 excision out of the host genome. Thus, Gp3-φBT1 and Gp3-φC31 are interchangeable in both in vitro and in vivo recombination; this could be a major concern when combining these two systems for genetic manipulation.
In conclusion, we have demonstrated that the phage-encoded protein, Gp3, is the RDF which controls the directionality of the reaction in φBT1 integrase-mediated site-specific recombination; and this function is realized by a protein-protein interaction with the integrase rather than direct binding to the substrates. Furthermore, the φBT1 integration system has been widely used for genetic engineering both in vivo [32], [33], [34], [35] and in vitro [22], [23], [36]; thus identification of the RDF (Gp3) reported here, could extend the potential utility of the φBT1 recombination system [32].
Supporting Information
Contains: Table S1, Table S2, Figure S1, Figure S2, Figure S3, Supplementary References.
(DOCX)
Acknowledgments
The authors thank members of the Zhao and Ding laboratories for their help and advice.
Funding Statement
This work was supported by National Basic Research Program of China (973 Program) (2012CB721102), National Natural Science Foundation of China (30830002), and the China Postdoctoral Science Foundation funded project (2012T50444 and 2012M520947). Websites: National Basic Research Program of China (http://www.973.gov.cn/English/Index.aspx), National Natural Science Foundation of China (http://www.nsfc.gov.cn/e_nsfc/desktop/zn/0101.htm), China Postdoctoral Science Foundation (http://res.chinapostdoctor.org.cn/Program/Main.html). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Khaleel T, Younger E, McEwan AR, Varghese AS, Smith MC (2011) A phage protein that binds φC31 integrase to switch its directionality. Mol Microbiol 80: 1450–1463. [DOI] [PubMed] [Google Scholar]
- 2.Kieser T, Bibb MJ, Buttner MJ, Chater KF, Hopwood DA (2000) Practical Streptomyces Genetics. Norwich, United Kingdom: The John Innes Foundation. [Google Scholar]
- 3. Ghosh P, Wasil LR, Hatfull GF (2006) Control of phage Bxb1 excision by a novel recombination directionality factor. PLoS Biol 4: 964–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bibb LA, Hancox MI, Hatfull GF (2005) Integration and excision by the large serine recombinase φRv1 integrase. Mol Microbiol 55: 1896–1910. [DOI] [PubMed] [Google Scholar]
- 5. Grindley NDF, Whiteson KL, Rice PA (2006) Mechanisms of site-specific recombination. AnnuRev Biochem 75: 567–605. [DOI] [PubMed] [Google Scholar]
- 6. Groth AC, Calos MP (2004) Phage integrases: Biology and applications. J Mol Biol 335: 667–678. [DOI] [PubMed] [Google Scholar]
- 7. Lewis JA, Hatfull GF (2001) Control of directionality in integrase-mediated recombination: examination of recombination directionality factors (RDFs) including Xis and Cox proteins. Nucleic Acids Res 29: 2205–2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Biswas T, Aihara H, Radman-Livaja M, Filman D, Landy A, et al. (2005) A structural basis for allosteric control of DNA recombination by λ integrase. Nature 435: 1059–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Richet E, Abcarian P, Nash HA (1988) Synapsis of attachment sites during λ integrative recombination involves capture of a naked dna by a protein-DNA complex. Cell 52: 9–17. [DOI] [PubMed] [Google Scholar]
- 10. Abremski K, Gottesman S (1981) Site-Specific Recombination: Xis-independent excisive recombination of bacteriophage λ. J Mol Biol 153: 67–78. [DOI] [PubMed] [Google Scholar]
- 11. Hsu PL, Ross W, Landy A (1980) The λ-phage att Site: Functional limits and interaction with Int protein. Nature 285: 85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kikuchi Y, Nash HA (1979) Nicking-closing activity associated with bacteriophage λ int gene product. Proc Natl Acad Sci USA 76: 3760–3764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Abbani MA, Papagiannis CV, Sam MD, Cascio D, Johnson RC, et al. (2007) Structure of the cooperative Xis-DNA complex reveals a micronucleoprotein filament that regulates phage λ intasome assembly. Proc Natl Acad Sci USA 104: 2109–2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hsu PL, Landy A (1984) Resolution of synthetic att-site Holliday structures by the integrase protein of bacteriophage λ. Nature 311: 721–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang L, Zhao GP, Ding XM (2010) Site-specific recombination systems: mechanisms and applications. Scientia Sinica Vitae 40: 1090–1111. [Google Scholar]
- 16. Rajeev L, Malanowska K, Gardner JF (2009) Challenging a paradigm: the role of DNA homology in tyrosine recombinase reactions. Microbiol Mol Biol Rev 73: 300–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Boocock MR, Zhu XW, Grindley NDF (1995) Catalytic residues of γδ resolvase act in cis . EMBO J14: 5129–5140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li WK, Kamtekar S, Xiong Y, Sarkis GJ, Grindley NDF, et al. (2005) Structure of a synaptic γδ resolvase tetramer covalently linked to two cleaved DNAs. Science 309: 1210–1215. [DOI] [PubMed] [Google Scholar]
- 19. Smith MC, Thorpe HM (2002) Diversity in the serine recombinases. Mol Microbiol 44: 299–307. [DOI] [PubMed] [Google Scholar]
- 20. Rowley PA, Smith MCA, Younger E, Smith MCM (2008) A motif in the C-terminal domain of φC31 integrase controls the directionality of recombination. Nucleic Acids Res 36: 3879–3891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Thorpe HM, Smith MCM (1998) In vitro site-specific integration of bacteriophage DNA catalyzed by a recombinase of the resolvase/invertase family. Proc Natl Acad Sci USA 95: 5505–5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhang L, Wang L, Wang J, Ou X, Zhao G, et al. (2010) DNA cleavage is independent of synapsis during Streptomyces phage φBT1 integrase-mediated site-specific recombination. J Mol Cell Biol 2: 264–275. [DOI] [PubMed] [Google Scholar]
- 23. Zhang L, Ou XJ, Zhao GP, Ding XM (2008) Highly efficient in vitro site-specific recombination system based on Streptomyces phage φBT1 integrase. J Bacteriol 190: 6392–6397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Morita K, Yamamoto T, Fusada N, Komatsu M, Ikeda H, et al. (2009) In vitro characterization of the site-specific recombination system based on actinophage TG1 integrase. Mol Genet Genomics 282: 607–616. [DOI] [PubMed] [Google Scholar]
- 25. Ghosh P, Kim AI, Hatfull GF (2003) The orientation of mycobacteriophage Bxb1 integration is solely dependent on the central dinucleotide of attP and attB . Mol Cell 12: 1101–1111. [DOI] [PubMed] [Google Scholar]
- 26. Smith MCA, Till R, Smith MCM (2004) Switching the polarity of a bacteriophage integration system. Mol Microbiol 51: 1719–1728. [DOI] [PubMed] [Google Scholar]
- 27. Breuner A, Brondsted L, Hammer K (1999) Novel organization of genes involved in prophage excision identified in the temperate lactococcal bacteriophage TP901-1. J Bacteriol 181: 7291–7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bai H, Sun M, Ghosh P, Hatfull GF, Grindley ND, et al. (2011) Single-molecule analysis reveals the molecular bearing mechanism of DNA strand exchange by a serine recombinase. Proc Natl Acad Sci USA 108: 7419–7424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Olorunniji FJ, Buck DE, Colloms SD, McEwan AR, Smith MC, et al. (2012) Gated rotation mechanism of site-specific recombination by φC31 integrase. Proc Natl Acad Sci USA 109: 19661–19666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Johnson RC, McLean MM (2011) Recombining DNA by protein swivels. Structure 19: 751–753. [DOI] [PubMed] [Google Scholar]
- 31. Gregory MA, Till R, Smith MCM (2003) Integration site for Streptomyces phage φBT1 and development of site-specific integrating vectors. J Bacteriol 185: 5320–5323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Baltz RH (2012) Streptomyces temperate bacteriophage integration systems for stable genetic engineering of actinomycetes (and other organisms). J Ind Microbiol Biotechnol 39: 661–672. [DOI] [PubMed] [Google Scholar]
- 33. Alexander DC, Rock J, He X, Brian P, Miao V, et al. (2010) Development of a genetic system for combinatorial biosynthesis of lipopeptides in Streptomyces fradiae and heterologous expression of the A54145 biosynthesis gene cluster. Appl Environ Microbiol 76: 6877–6887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Liu H, Jiang H, Haltli B, Kulowski K, Muszynska E, et al. (2009) Rapid cloning and heterologous expression of the meridamycin biosynthetic gene cluster using a versatile Escherichia coli-Streptomyces artificial chromosome vector, pSBAC. J Nat Prod 72: 389–395. [DOI] [PubMed] [Google Scholar]
- 35. Xu Z, Lee NCO, Dafhnis-Calas F, Malla S, Smith MCM, et al. (2008) Site-specific recombination in Schizosaccharomyces pombe and systematic assembly of a 400 kb transgene array in mammalian cells using the integrase of Streptomyces phage φBT1. Nucleic Acids Res 36: E9–E9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhang L, Zhao GP, Ding XM (2011) Tandem assembly of the epothilone biosynthetic gene cluster by in vitro site-specific recombination. Sci Rep 1: 141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Datsenko KA, Wanner BL (2000) One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci USA 97: 6640–6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Davies GE, Stark GR (1970) Use of dimethyl suberimidate, a cross-linking reagent, in studying subunit structure of oligomeric proteins. Proc Natl Acad Sci USA 66: 651–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Stark WM (2011) Cutting out the φC31 prophage. Mol Microbiol 80: 1417–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ghosh P, Bibb LA, Hatfull GF (2008) Two-step site selection for serine-integrase-mediated excision: DNA-directed integrase conformation and central dinucleotide proofreading. Proc Natl Acad Sci USA 105: 3238–3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Morita K, Yamamoto T, Fusada N, Komatsu M, Ikeda H, et al. (2009) The site-specific recombination system of actinophage TG1. FEMS Microbiol Lett 297: 234–240. [DOI] [PubMed] [Google Scholar]
- 42. Savinov A, Pan J, Ghosh P, Hatfull GF (2012) The Bxb1 gp47 recombination directionality factor is required not only for prophage excision, but also for phage DNA replication. Gene 495: 42–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Contains: Table S1, Table S2, Figure S1, Figure S2, Figure S3, Supplementary References.
(DOCX)