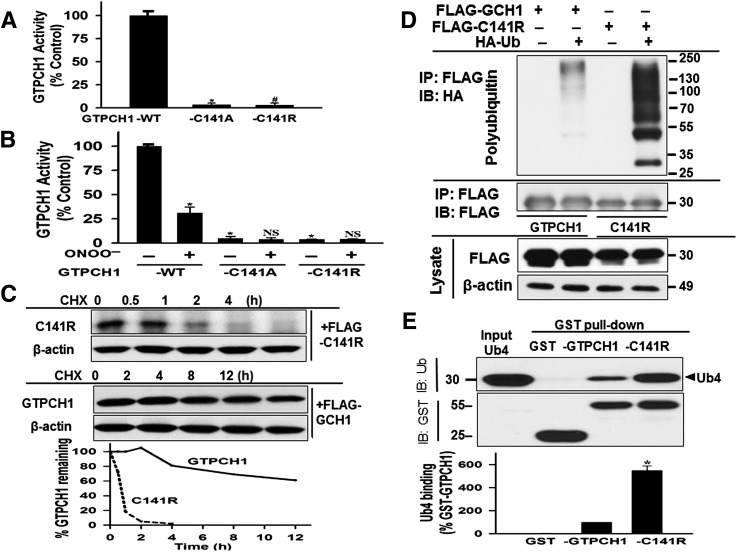
FIG. 3.
Genetic deletion of zinc from GTPCH1 impairs its activity and accelerates GTPCH1 degradation and ubiquitination via direct ubiquitin binding. A: Enzyme activity of recombinant GTPCH1 and zinc-deleted GTPCH1 (C141A and C141R). FLAG-tagged WT or C141A or C141R GTPCH1 plasmids were constructed and expressed in HEK293 cells as described in research design and methods. Recombinant WT GTPCH1 or the C141A or C141R mutants were purified by anti-FLAG resin and subjected to activity assay by HPLC. #, n = 3. *P < 0.05 vs. control. B: Effects of ONOO− on GTPCH1 activity of GTPCH1 WT, C141A, or C141R mutant. *P < 0.05 when compared to vehicle-treated WT. C: Representative blots of three independent experiments showing the protein stability of C141R. HEK293 cells were transfected with FLAG-C141R or -GTPCH1 plasmids. Twenty-four hours after transfection, cells were incubated with CHX (100 μg/mL), and, at the indicated times, cells were harvested, lysed, and analyzed for GTPCH1 levels by using an anti-FLAG antibody. In all cases, β-actin levels were measured as a loading control. D: C141R ubiquitination in cultured cells. HEK293 cells were cotransfected with FLAG-tagged WT or C141R GTPCH1, and HA-tagged ubiquitin plasmid or empty vector. Cell lysates were immunoprecipitated by anti-FLAG resin and immunoblotted using the indicated antibodies. E: Effects of C141R ubiquitination in vitro. Purified recombinant GST-GTPCH1 (WT) or GST-C141R proteins were incubated with Ub4. Bound ubiquitin chains were separated by SDS-PAGE and analyzed by immunoblotting. The blot shown is representative of three independent experiments. Data are shown as mean ± SD (n = 3). *P < 0.05 vs. control. NS, not significant; IB, immunoblot; IP, coimmunoprecipitation; Ub, ubiquitin.