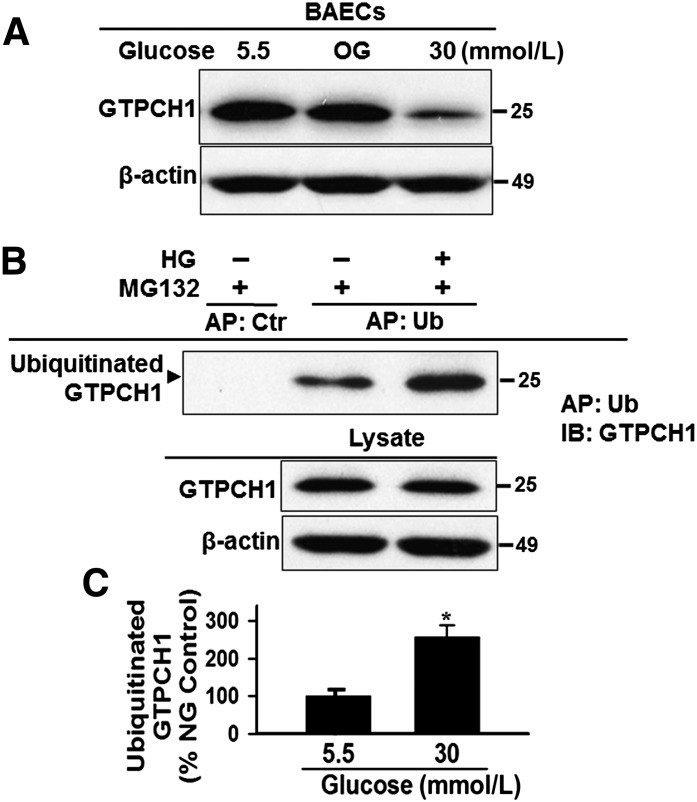
FIG. 6.
High glucose increases GTPCH1 ubiquitination and degradation. A: High glucose increases GTPCH1 degradation. Confluent BAECs were treated with normal-glucose (NG) medium (d-glucose, 5 mmol/L), osmotic glucose (OG; d-glucose 5 mmol/L, l-glucose 25 mmol/L), or high glucose (HG; d-glucose, 30 mmol/L), and the cells were harvested, lysed, and analyzed for GTPCH1 levels by immunoblotting. B and C: High glucose increases GTPCH1 ubiquitination. BAECs were pretreated with MG132 (0.5 μmol/L) for 30 min and treated with NG medium (d-glucose, 5 mmol/L) or HG (d-glucose, 30 mmol/L) in the presence of MG132 (0.5 μmol/L) for 6 h. Cell lysates were incubated with ubiquitin interacting motif-agarose for ubiquitin (Ub) AP. Ubiquitinated proteins were analyzed by immunoblotting with anti-GTPCH1 and anti–β-actin antibodies. The blot shown is representative of three independent experiments. IB, immunoblot. *P < 0.05 vs. 5.5 mmol/L.