Bile acids (BA) are amphipathic molecules whose physicochemical properties facilitate the solubilization of biliary cholesterol and intestinal lipids. As detergents, excessive concentrations of BA, such as in cholestatic diseases, are toxic, imposing a tight control of BA pool size and in particular its hydrophobicity level. Hence, BA synthesis is under negative control by BA themselves, which act as signaling molecules via specific pathways, including the G protein–coupled BA receptor (TGR5) and the nuclear receptors farnesoid X receptor (FXR), vitamin D receptor, and pregnane X receptor as well as different kinase signaling pathways. Recently, BA were identified as regulators of glucose, lipid, and energy metabolism with unexpected roles for TGR5 and FXR therein (1).
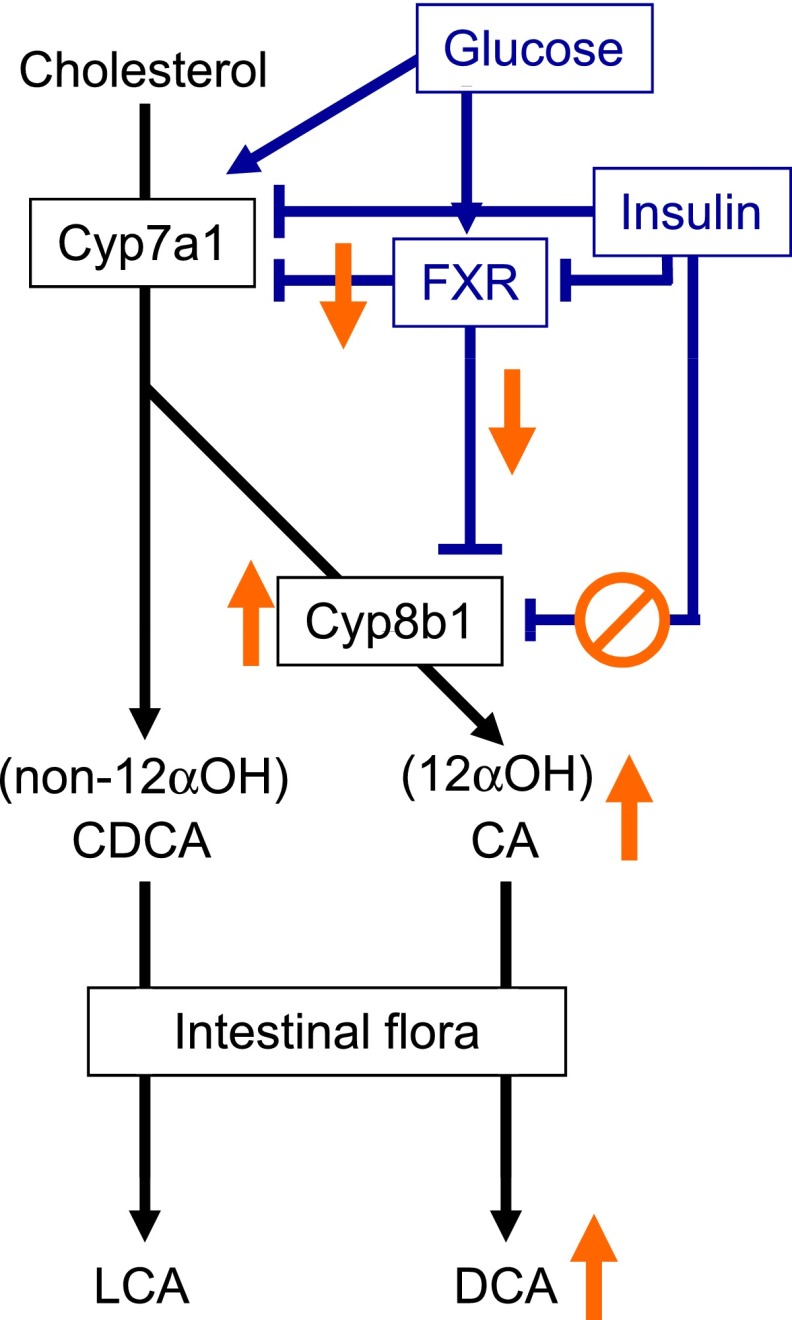
BA are synthesized from cholesterol by the hepatic parenchymal cells through the classical and alternative pathways with cholesterol 7 α-hydroxylase (Cyp7a1) and cholesterol 27 α-hydroxylase (Cyp27a1) as first enzymes, respectively. In humans, the ratio of the primary BA cholic acid (CA)/chenodeoxycholic acid (CDCA) determines the hydrophobicity of the BA pool, a step regulated by the activity of cholesterol 12α-hydroxylase (Cyp8b1), which adds an additional OH group at C12 (Fig. 1). CDCA and CA are conjugated to glycine or taurine and secreted via the bile canaliculus into the intestine, where they are transformed through dehydroxylation by the intestinal flora into the more hydrophobic secondary BA lithocholic acid (LCA) and deoxycholic acid (DCA), respectively, before returning to the liver via the enterohepatic cycle. The expression and activity of the hepatic BA synthesis enzymes is negatively controlled by BA acting via FXR in both intestine (via FGF19 induction) and liver (via the short heterodimer partner/liver receptor homolog-1 pathway). Moreover, studies in rodents have identified that both BA pool size and composition are dependent on the metabolic status, being, among other factors, regulated by insulin (2). Although of mechanistic interest, studies in rodents provide limited information on BA metabolism in humans, since the murine BA pool composition is strikingly different with CDCA being further converted to more hydrophilic muricholic acid species. Hence, since Cyp8b1 has opposite effects on the BA hydrophobicity index between humans and rodents, a proper assessment of its functional role and the different BA species in the control of metabolism requires translational studies in humans.
FIG. 1.
Schematic overview of bile acid synthesis and its regulation. Changes in insulin resistance and/or type 2 diabetes are indicated in orange. 12αOH, 12α-hydroxylated BA.
In the study published in this issue, Haeusler et al. (3) performed a thorough characterization of the content and composition of blood BA in healthy subjects and diabetic patients and performed correlations with parameters of glucose homeostasis. In 200 apparently healthy subjects, the degree of insulin sensitivity, measured by hyperinsulinemic-euglycemic clamp, correlated with the composition rather than the size of the BA pool. The authors found a significant association between the ratio of 12α-hydroxylated/non–12α-hydroxylated BA (represented predominantly by CA+DCA/CDCA) and the degree of insulin resistance, suggesting a higher activity of Cyb8B1 in the most insulin-resistant subjects. By contrast, there was no association with the ratio of unconjugated/conjugated, secondary/primary, and total plasma BA concentrations. Subsequent principal component analysis identified a relationship of the 12α-hydroxylated/non–12α-hydroxylated BA ratio and most metabolic parameters associated with insulin resistance, such as BMI, fasting, and postglucose insulin secretion, several liver function parameters, plasma triglycerides, and HDL. Interestingly, total plasma BA concentrations rather correlated with changes in amino acid and fat metabolism than with glucose metabolism. Quite surprisingly, the picture was different in diabetic patients, who displayed elevated total plasma BA concentrations and a higher hydrophobicity index, but no disproportionate increase in 12α-hydroxylated BA.
This study is original in that it provides detailed data on the relationship between plasma BA and parameters of glucose homeostasis in a relatively large human cohort, comparing individuals with different degrees of insulin resistance to patients with established diabetes. As indicated by Haeusler et al. (3), a major limitation of such an association study resides in its correlative nature not allowing conclusions on causality or the identification of the mechanisms involved. For this purpose tracer kinetic studies are required, preferentially combined with analysis of tissue biopsies and genetic variations, which unfortunately are inherently difficult to perform in humans.
How do alterations in glucose metabolism and insulin signaling influence BA pool size and composition? Based on their previous mechanistic studies in rodents (4), the authors suggest the involvement of FoxO1, a transcription factor that induces Cyp8b1 expression and whose activity is negatively controlled by insulin. In insulin resistance, this inhibition might fall short leading to an increased synthesis of 12α-hydroxylated BA. Although the authors’ suggestion is plausible, several mechanisms likely act in concert. FXR, which represses both Cyp7a1 and Cyp8b1 expression, is itself regulated by insulin (negatively) and glucose (positively) fluxes at gene expression (5) and posttranslational levels (6), possibly contributing to the dysregulation of BA synthesis in insulin resistance and diabetes. In addition, hepatic insulin resistance could increase BA synthesis via a derepression of Cyp7a1 activity in patients with diabetes (7,8). Finally, recent studies associated the intestinal flora with changes in glucose homeostasis, BA metabolism, and hepatic FXR activity (9). In line, the authors observed a progressive increase of the secondary BA DCA, the major component of the 12α-hydroxylated BA pool, with increasing insulin resistance and diabetes. Consistently, Brufau et al. (10) showed an increased DCA input rate in type 2 patients with diabetes, likely reflecting altered intestinal flora activity. Thus, it would be interesting to determine the contribution of changes in the intestinal flora to BA profile differences.
Do the changes in BA metabolism, in turn, impact on glucose metabolism or are BA innocent bystander markers of the metabolic state? Although a correlative study cannot answer this important question, several lines of evidence suggest that BA directly modulate glucose metabolism via receptor-mediated mechanisms. BA-activated TGR5 induces glucagon-like peptide 1 secretion from intestinal L cells (11). Moreover, FXR modulates the kinetics of intestinal glucose absorption (12) and hepatic glycolysis (13). A recent study further proposed that BA signal in a hepatic–pancreatic axis, communicating changes in hepatic glucose sensing to the pancreas to adapt insulin secretion (14). Most compelling data from humans demonstrating the metabolic impact of BA pool manipulation come from treatment with BA sequestrants, which interrupt the enterohepatic cycle and not only lower plasma cholesterol but also improve glycemic control in diabetic patients (15). An intriguing consequence of the present findings is that changes in BA pool composition in insulin resistance may shift the ratio of agonistic and antagonistic ligands for BA receptors, resulting in altered activities of FXR and TGR5 on glucose and lipid metabolism. As such, a (relative) decrease in FXR activity could contribute to the increase in plasma triglycerides due to a less efficient inhibition of lipogenesis (16). Moreover, changes in BA pool composition may also, via its physicochemical properties, influence the intestinal absorption of cholesterol, triglycerides, and glucose and, as such, contribute to the correlations between BA pool composition, triglycerides, and HDL.
Haeusler et al. (3) conclude that manipulation of BA composition may constitute an approach to develop insulin-sensitizing therapeutics. Although the study does not provide causal evidence, studies in animal models have also suggested that inhibition of Cyp8b1could be an approach to control lipid absorption, cholesterol homeostasis, and atherosclerosis (17). In conclusion, this elegant study adds another piece to the puzzle tightening the link between BA and glucose metabolism in humans and warrants further investigations to determine whether targeting BA signaling pathways constitutes a viable pharmacological option in the treatment of type 2 diabetes and its complications.
ACKNOWLEDGMENTS
No potential conflicts of interest relevant to this article were reported.
Footnotes
See accompanying original article, p. 4184.
REFERENCES
- 1.Porez G, Prawitt J, Gross B, Staels B. Bile acid receptors as targets for the treatment of dyslipidemia and cardiovascular disease. J Lipid Res 2012;53:1723–1737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prawitt J, Caron S, Staels B. Bile acid metabolism and the pathogenesis of type 2 diabetes. Curr Diab Rep 2011;11:160–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haeusler RA, Astiarraga B, Camastra S, Accili D, Ferrannini E. Human insulin resistance is associated with increased plasma levels of 12α hydroxylated bile acids. Diabetes 2013;62:4184–4191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haeusler RA, Pratt-Hyatt M, Welch CL, Klaassen CD, Accili D. Impaired generation of 12-hydroxylated bile acids links hepatic insulin signaling with dyslipidemia. Cell Metab 2012;15:65–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duran-Sandoval D, Mautino G, Martin G, et al. Glucose regulates the expression of the farnesoid X receptor in liver. Diabetes 2004;53:890–898 [DOI] [PubMed] [Google Scholar]
- 6.Gineste R, Sirvent A, Paumelle R, et al. Phosphorylation of farnesoid X receptor by protein kinase C promotes its transcriptional activity. Mol Endocrinol 2008;22:2433–2447 [DOI] [PubMed] [Google Scholar]
- 7.Park WH, Pak YK. Insulin-dependent suppression of cholesterol 7α-hydroxylase is a possible link between glucose and cholesterol metabolisms. Exp Mol Med 2011;43:571–579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Twisk J, Hoekman MF, Lehmann EM, Meijer P, Mager WH, Princen HM. Insulin suppresses bile acid synthesis in cultured rat hepatocytes by down-regulation of cholesterol 7 alpha-hydroxylase and sterol 27-hydroxylase gene transcription. Hepatology 1995;21:501–510 [PubMed] [Google Scholar]
- 9.Sayin SI, Wahlström A, Felin J, et al. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab 2013;17:225–235 [DOI] [PubMed] [Google Scholar]
- 10.Brufau G, Stellaard F, Prado K, et al. Improved glycemic control with colesevelam treatment in patients with type 2 diabetes is not directly associated with changes in bile acid metabolism. Hepatology 2010;52:1455–1464 [DOI] [PubMed] [Google Scholar]
- 11.Katsuma S, Hirasawa A, Tsujimoto G. Bile acids promote glucagon-like peptide-1 secretion through TGR5 in a murine enteroendocrine cell line STC-1. Biochem Biophys Res Commun 2005;329:386–390 [DOI] [PubMed] [Google Scholar]
- 12.van Dijk TH, Grefhorst A, Oosterveer MH, et al. An increased flux through the glucose 6-phosphate pool in enterocytes delays glucose absorption in Fxr-/- mice. J Biol Chem 2009;284:10315–10323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caron S, Huaman Samanez C, Dehondt H, et al. Farnesoid X receptor inhibits the transcriptional activity of carbohydrate response element binding protein in human hepatocytes. Mol Cell Biol 2013;33:2202–2211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seyer P, Vallois D, Poitry-Yamate C, et al. Hepatic glucose sensing is required to preserve β cell glucose competence. J Clin Invest 2013;123:1662–1676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Out C, Groen AK, Brufau G. Bile acid sequestrants: more than simple resins. Curr Opin Lipidol 2012;23:43–55 [DOI] [PubMed] [Google Scholar]
- 16.Watanabe M, Houten SM, Wang L, et al. Bile acids lower triglyceride levels via a pathway involving FXR, SHP, and SREBP-1c. J Clin Invest 2004;113:1408–1418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Slätis K, Gåfvels M, Kannisto K, et al. Abolished synthesis of cholic acid reduces atherosclerotic development in apolipoprotein E knockout mice. J Lipid Res 2010;51:3289–3298 [DOI] [PMC free article] [PubMed] [Google Scholar]