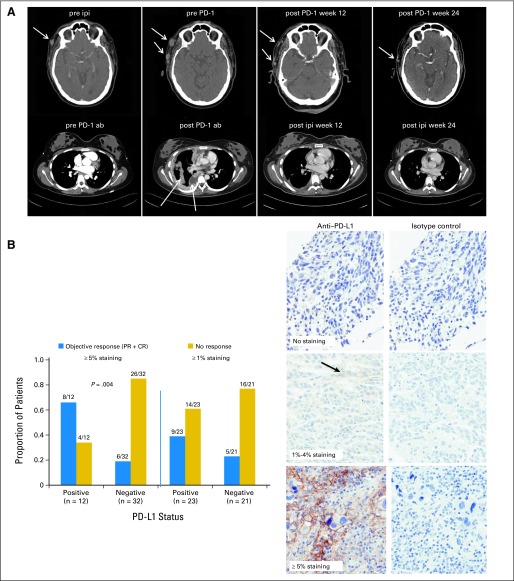
Fig 1.
(A) The upper row shows computed tomography (CT) scan images of the neck of a patient in cohort 4 who received ipilimumab at 3 mg/kg (pre ipi) and who experienced progression at week 12 with multiple subcutaneous lesions shown at the arrows (pre PD-1). Regression of disease occurred after six doses of nivolumab at 3 mg/kg at week 12 (post PD-1 week 12) and further regression at week 24 (post PD-1 week 24). The lower row shows CT scan images of the thorax of a patient in cohort 2 who received nivolumab 1 mg/kg (pre PD-1 ab) at baseline and who experienced progression at week 12 with increased pleural disease shown at the arrows (post PD-1 ab). There was significant regression of pleural and lung disease after four doses of ipilimumab at 3 mg/kg off protocol at week 12 (post ipi week 12) and further regression at week 24 (post ipi week 24). (B) Bar graphs indicating proportion of patients that are responders (blue bars) or nonresponders (yellow bars) using positive staining cutoffs of 1% or 5% tumor cells with membranous staining of 100 tumor cells counted to define programmed death-ligand 1 (PD-L1) status. Examples of zero staining and ≥ 1% and ≥ 5% positive staining of tumor cells are also shown in the near right column of three micrographs, with corresponding control images with isotype control staining shown in the far right column of three micrographs. Results for a total of 44 patients with available specimens are shown. CR, complete response; PD-1, programmed death-1; PR, partial response.