FIGURE 5.
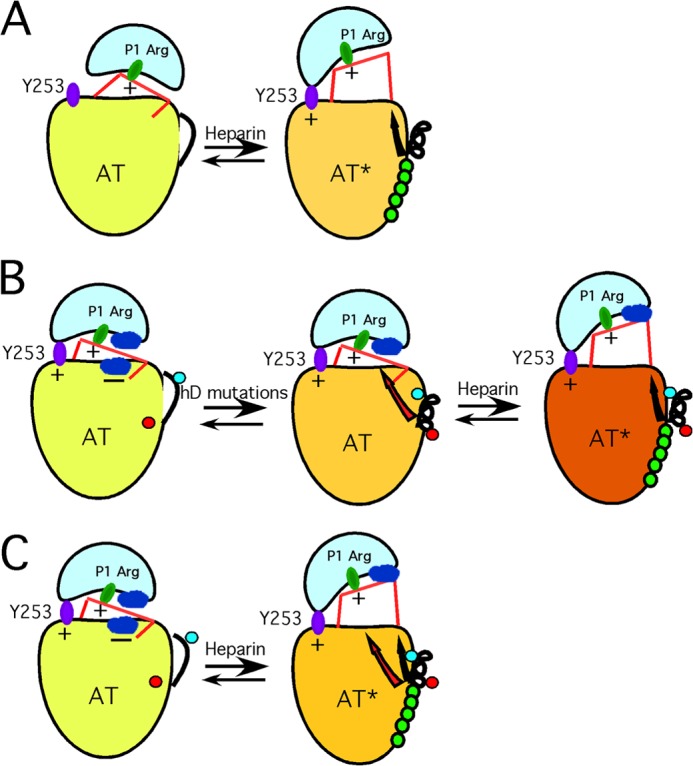
Proposed new model of antithrombin activation. A, schematic of the traditional view of how H5 results in activation of antithrombin as a factor IXa and factor Xa inhibitor. The low activity state (left), with inserted RCL hinge, reacts poorly because of the inability of the proteinase to dock with an exosite represented by Tyr-253. The sole mechanistic role of H5 binding is to extend helix D, thereby expelling the RCL hinge and allowing the proteinase (IXa or Xa) to engage the Tyr-253 exosite. B, schematic of how we envision the 131–136 mutations cause activation without the need for RCL hinge expulsion. The left-hand structure represents an altered view of how WT antithrombin is configured in a low activity state, based on evidence presented in an earlier publication (36). Here, low activity results not from an inability of the proteinase to engage the Tyr-253 exosite but from nonoptimal interactions at the interface between proteinase and the antithrombin surface (shown as blue patches on each protein and signified as unfavorable by a minus sign). Introduction of the 131–136 mutations (middle structure) leads to the following: (i) change in conformation of Tyr-131 (red dot) from buried to exposed, and (ii) extension of helix D such that the previously exposed Ala-134 (blue dot) is buried in the protein core. Together, these changes give rise to a long range conformational change (red arrow) sensed at the site of the unfavorable interaction between antithrombin and the proteinase, thereby giving rise to the massive activation that normally requires H5 binding. Because RCL hinge expulsion has been decoupled by introduction of the 131–136 mutations, H5 binding is required to give the final RCL hinge expulsion (black arrow) and results in a final small augmentation in rate (right structure). C, represents our new proposed mechanism of how H5 activates WT antithrombin, based on the findings of the 131–136 and related variants. Here, because RCL hinge expulsion and the other conformational changes are no longer decoupled, both sets of conformational changes are induced by H5 binding. Again, the reduction in unfavorable interactions (red arrow) results in most of the rate enhancement, although the RCL hinge expulsion (black arrow) may give additional small enhancement by allowing the proteinase to move further away from the antithrombin surface.
